作者/講者: 王舜禾 醫師
整理: 李耿列 醫師
校稿: Ian YC Chen, MD
上次校稿: 2018/03/29

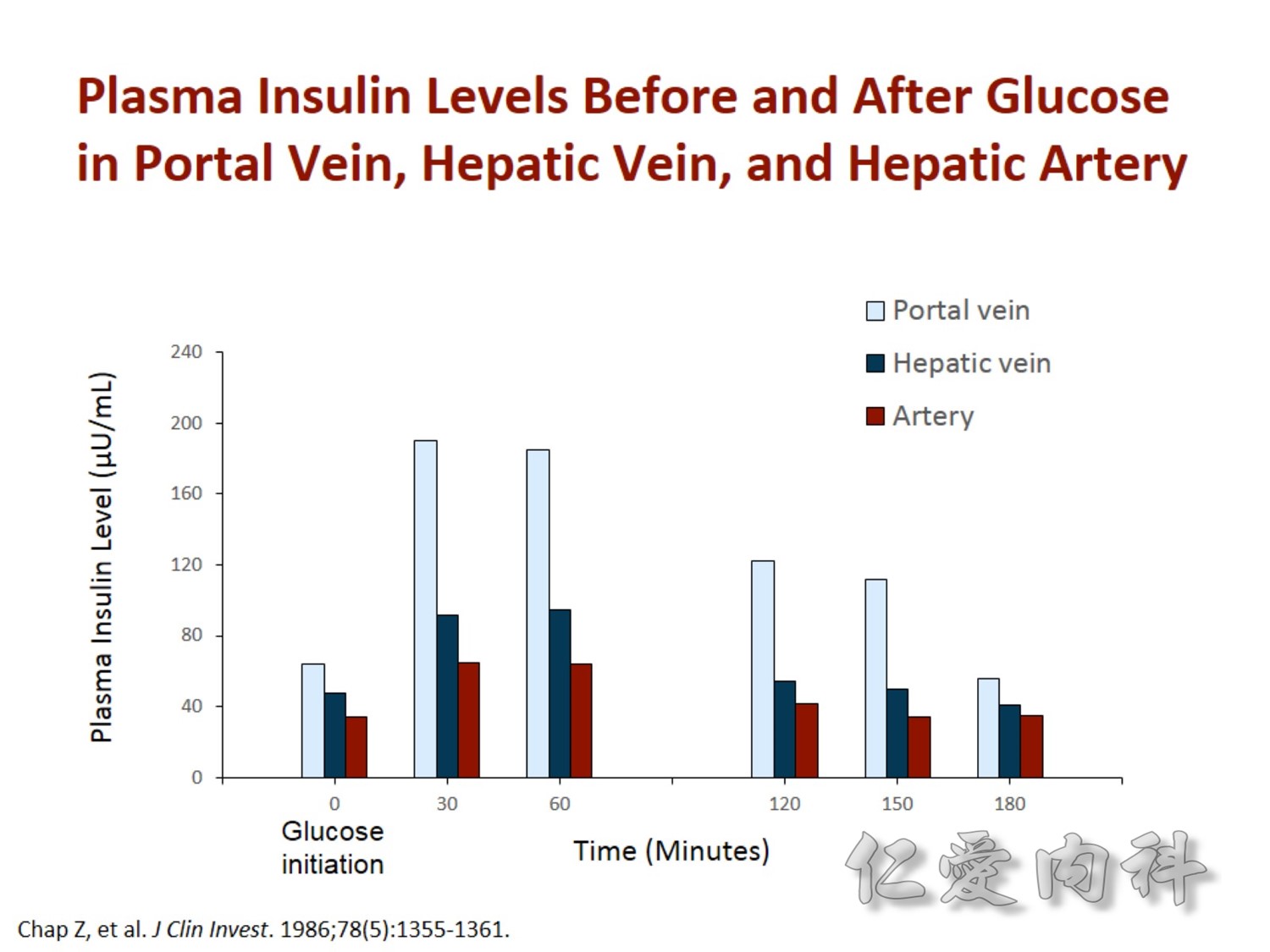
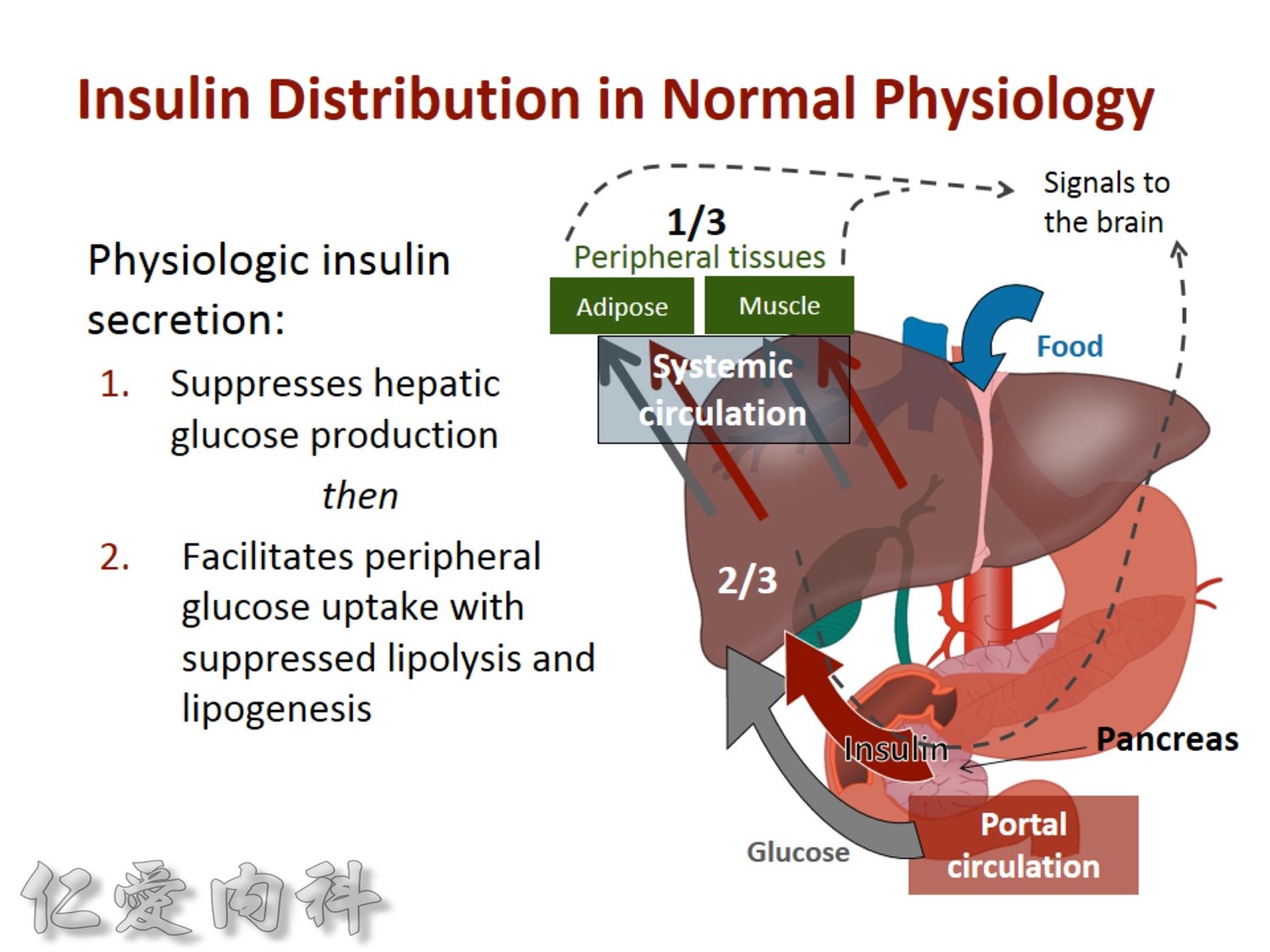
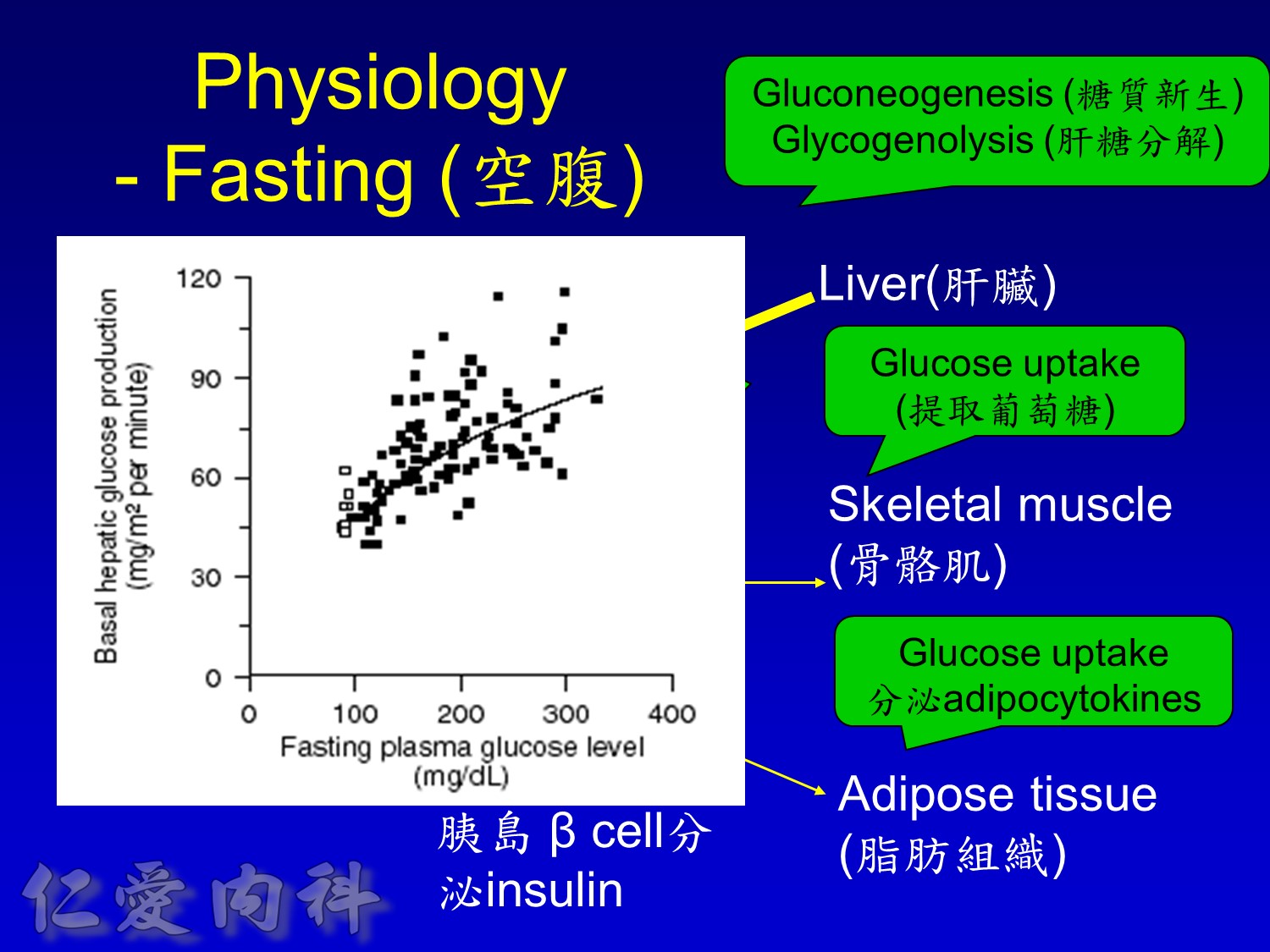
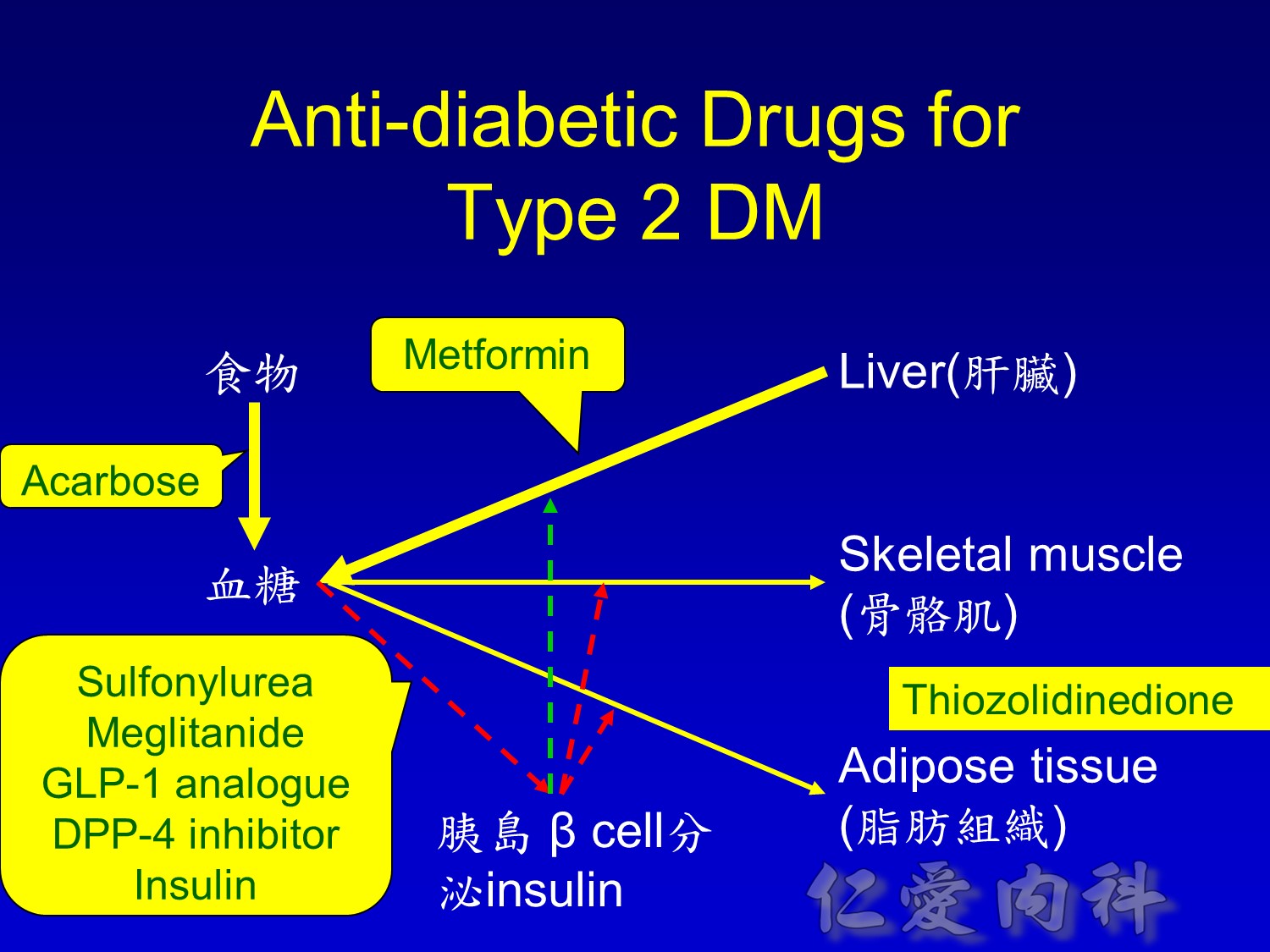
2/3作用在肝臟經腸肝循環portal vein ==> hepatic vein , thus gluconeogenesis will diminish
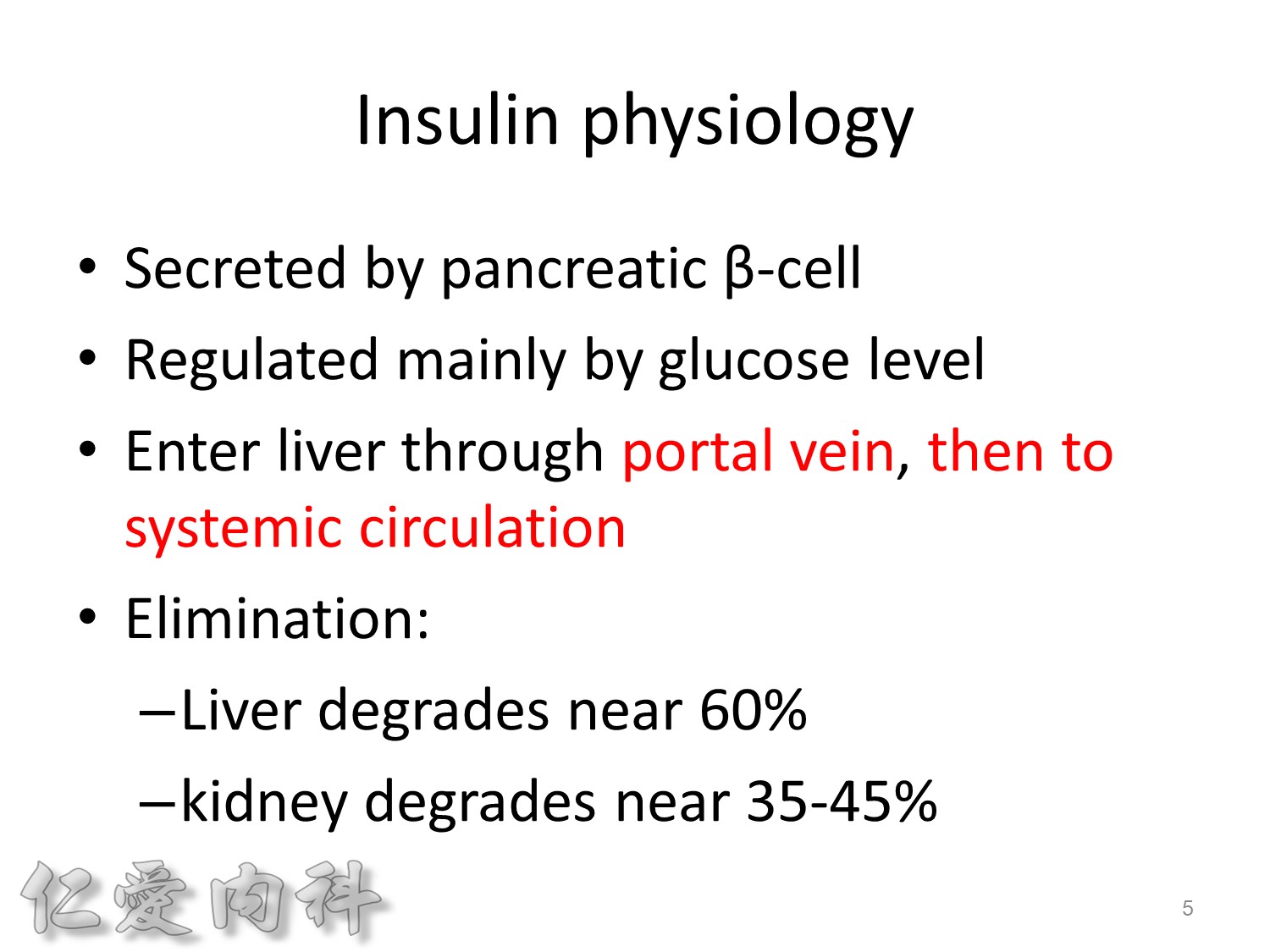
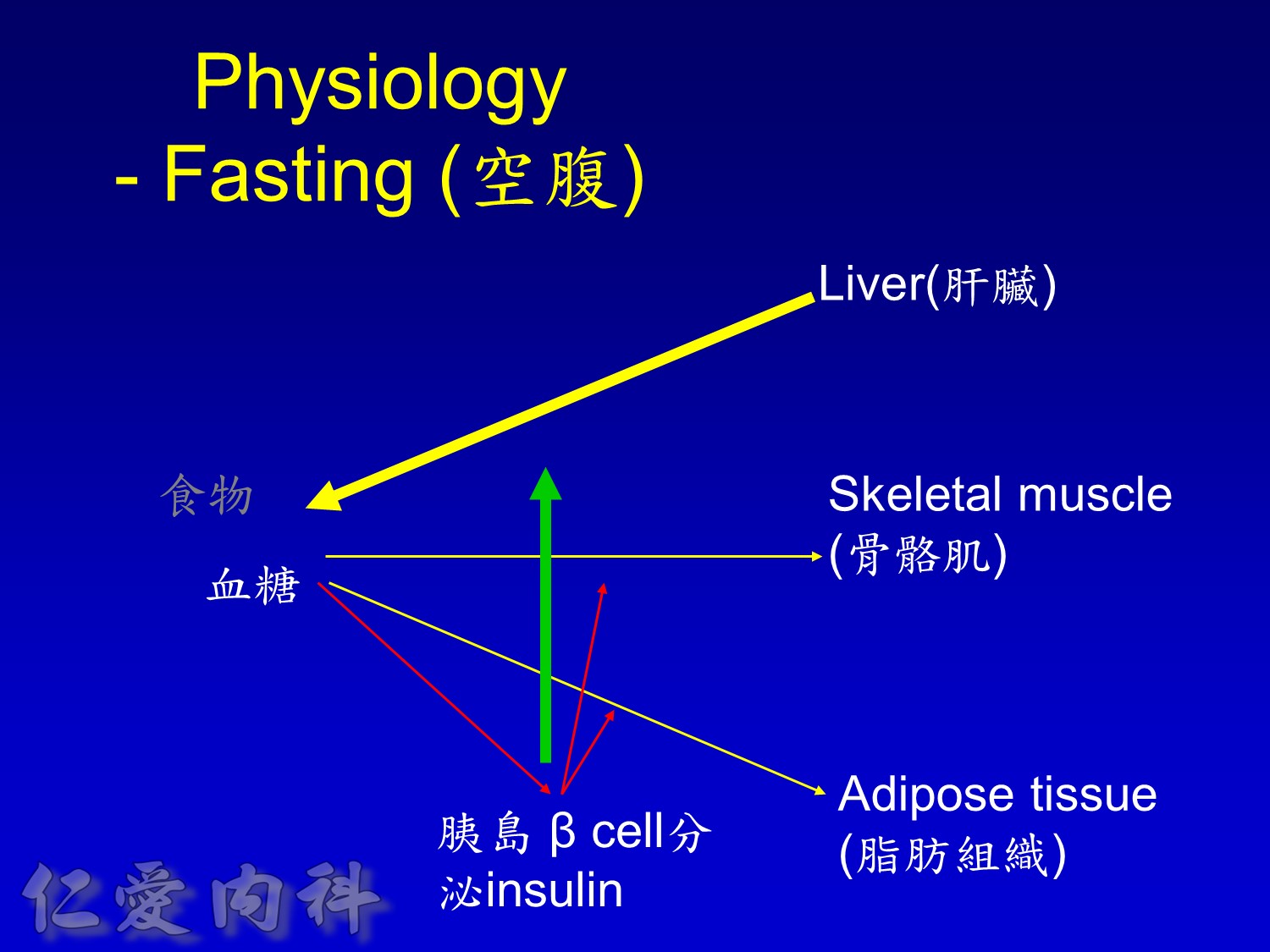
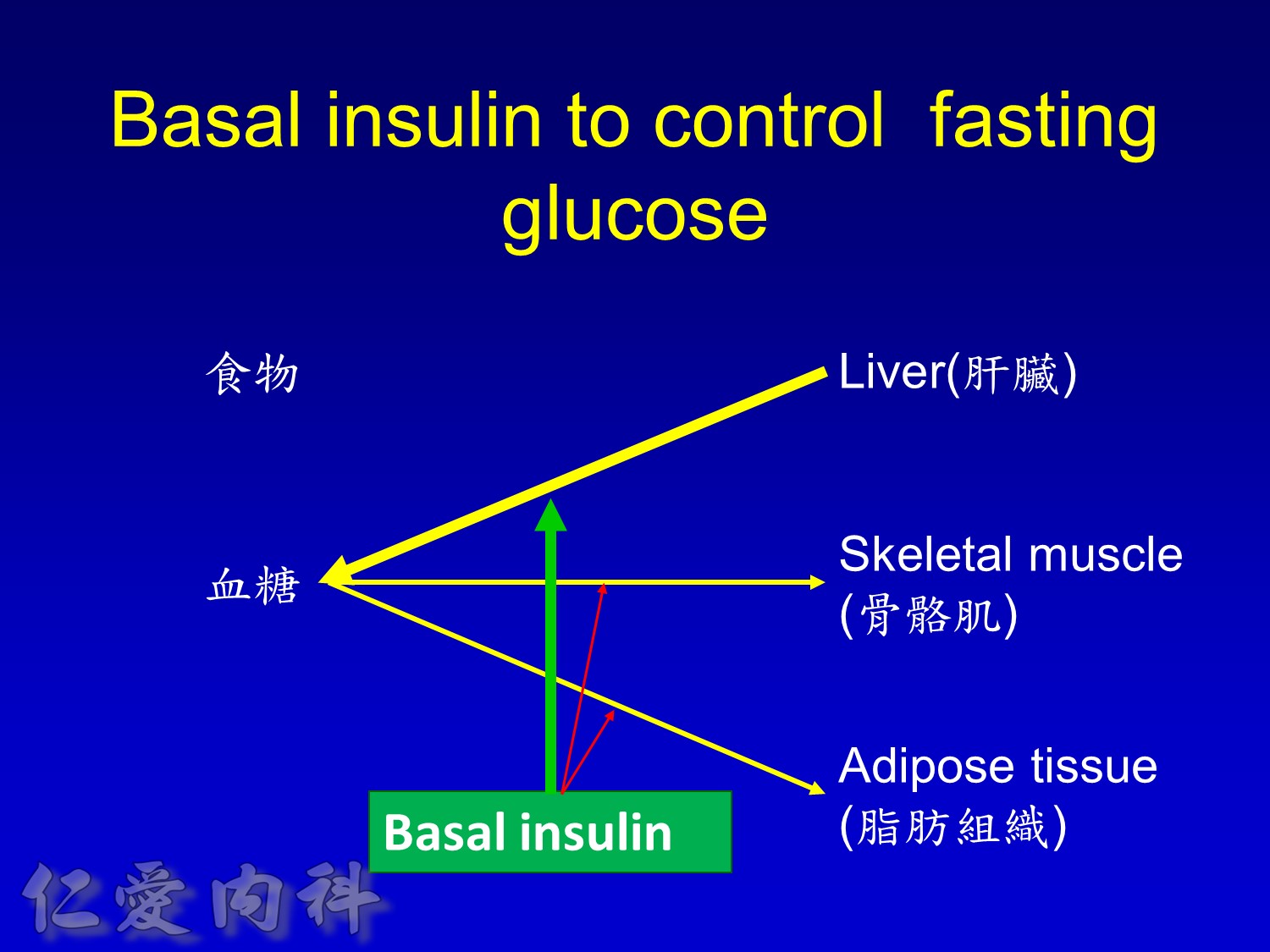
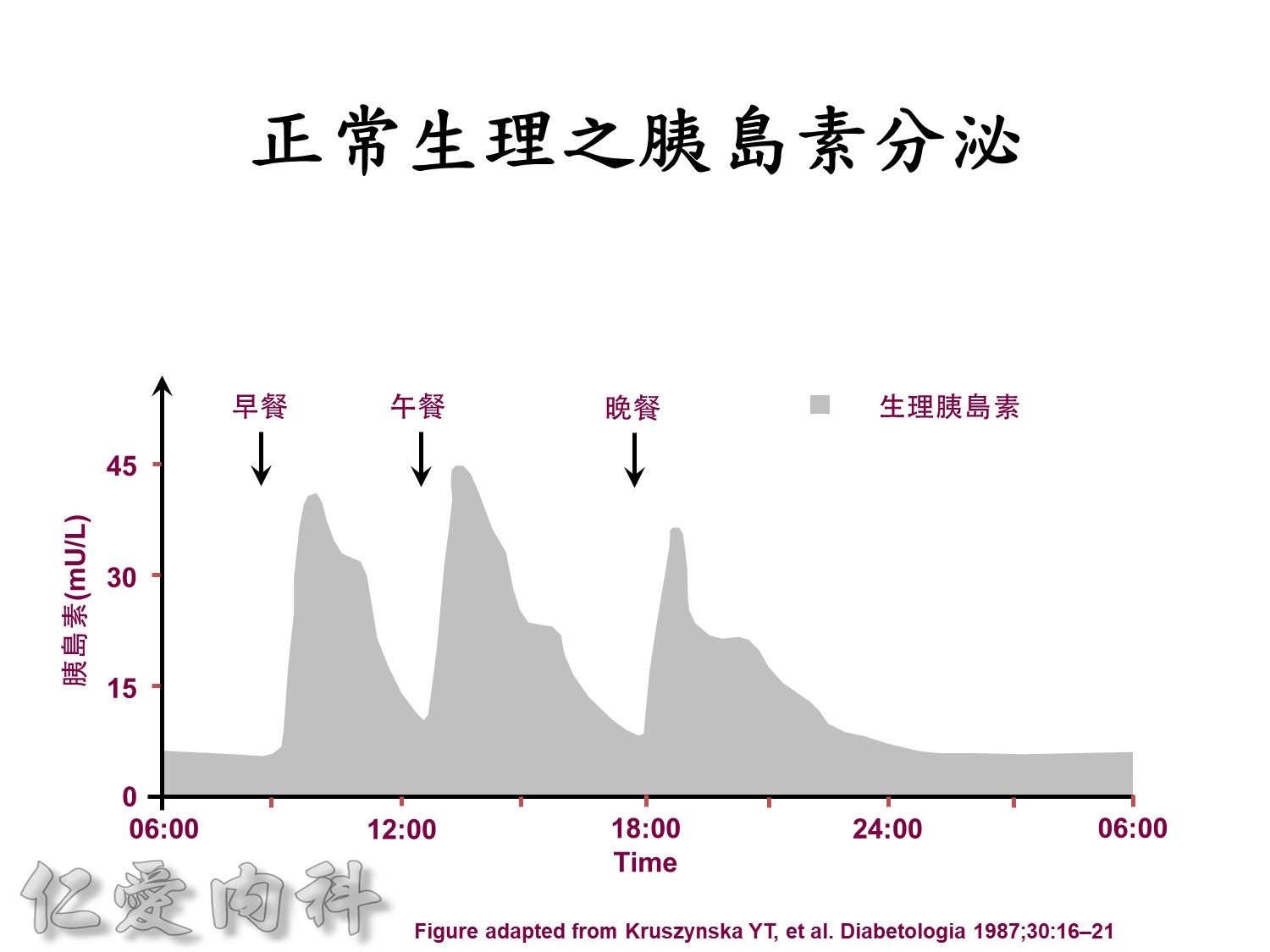
Basal insulin will secret even in fasting status
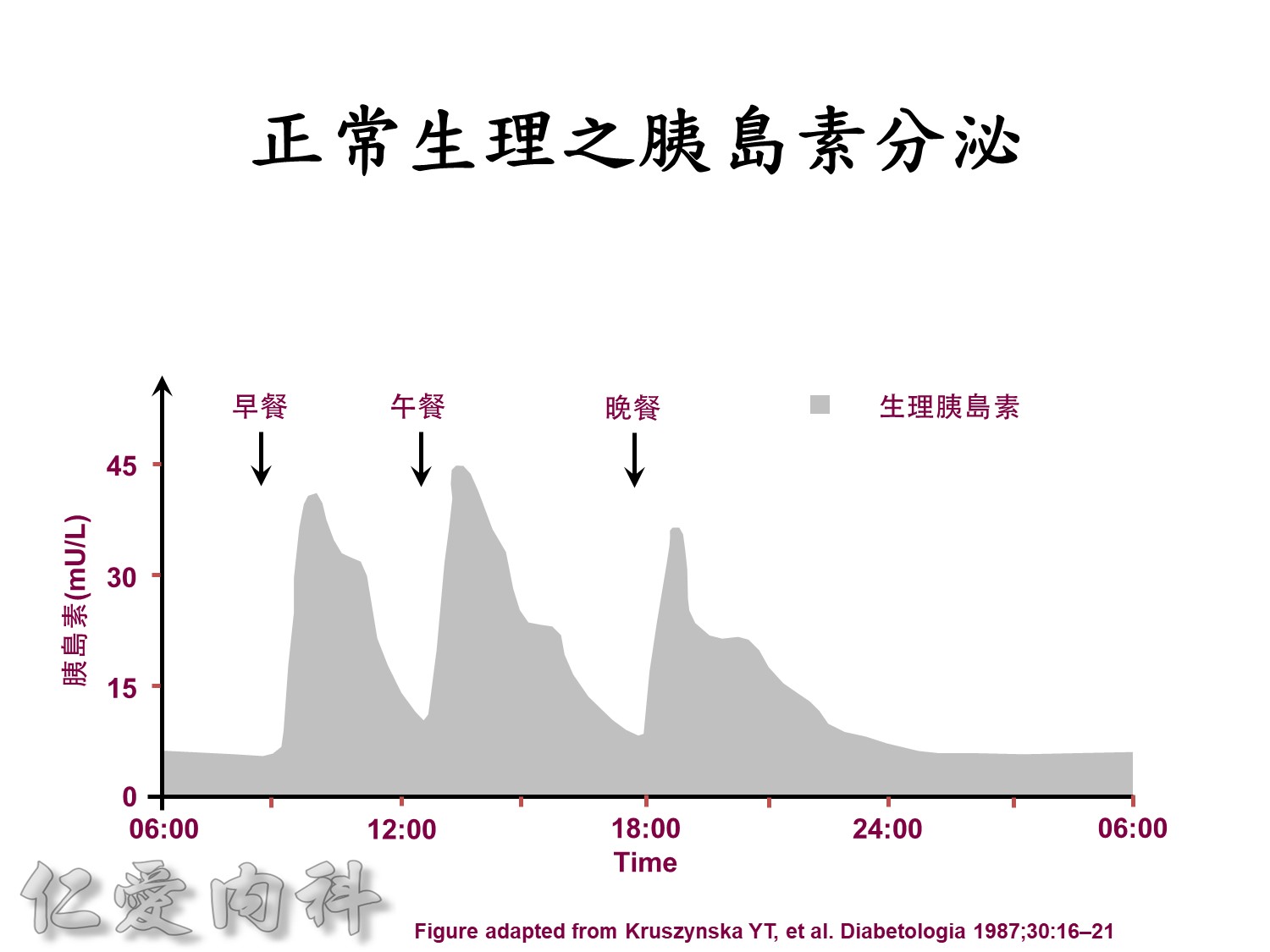
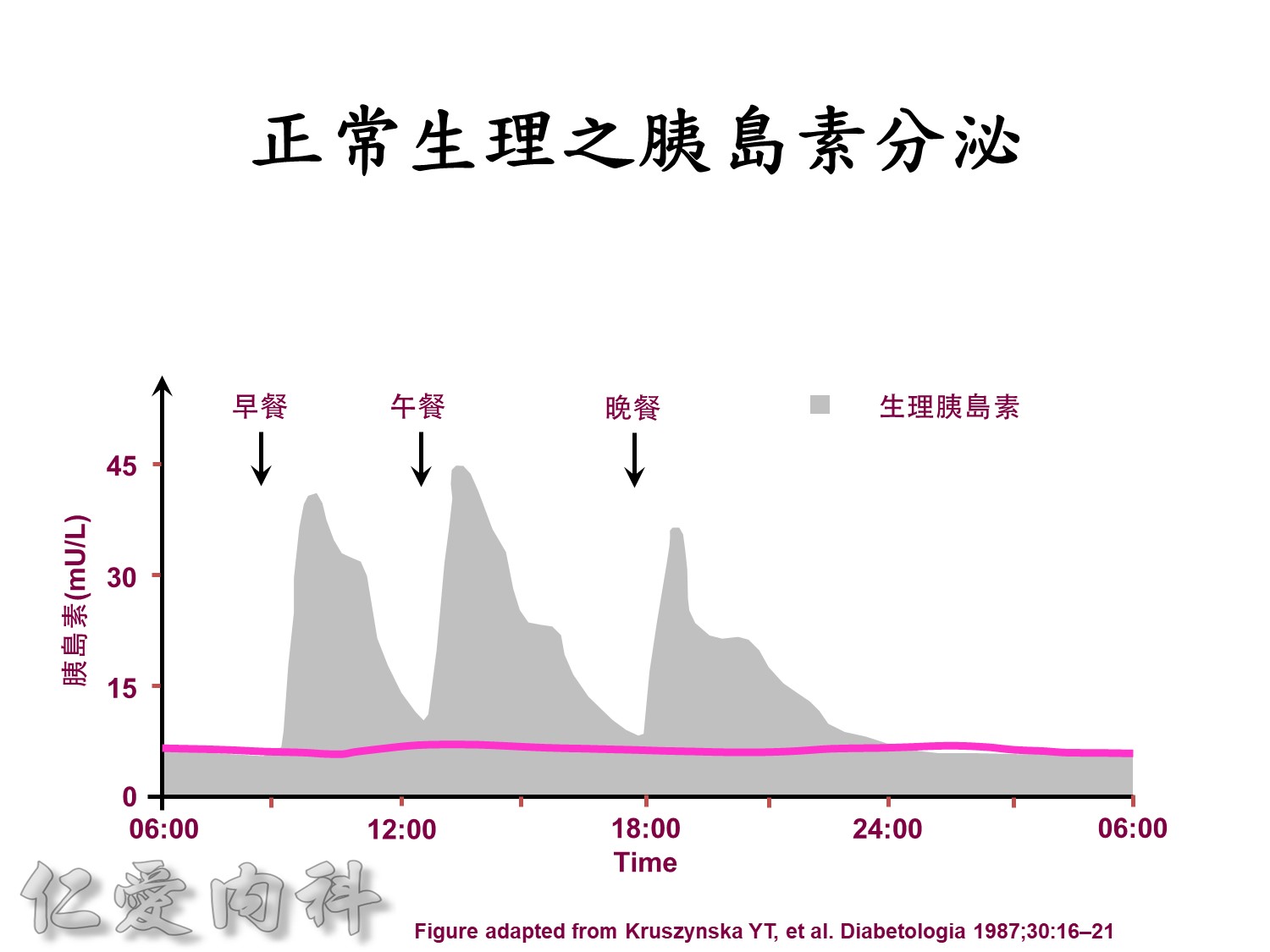

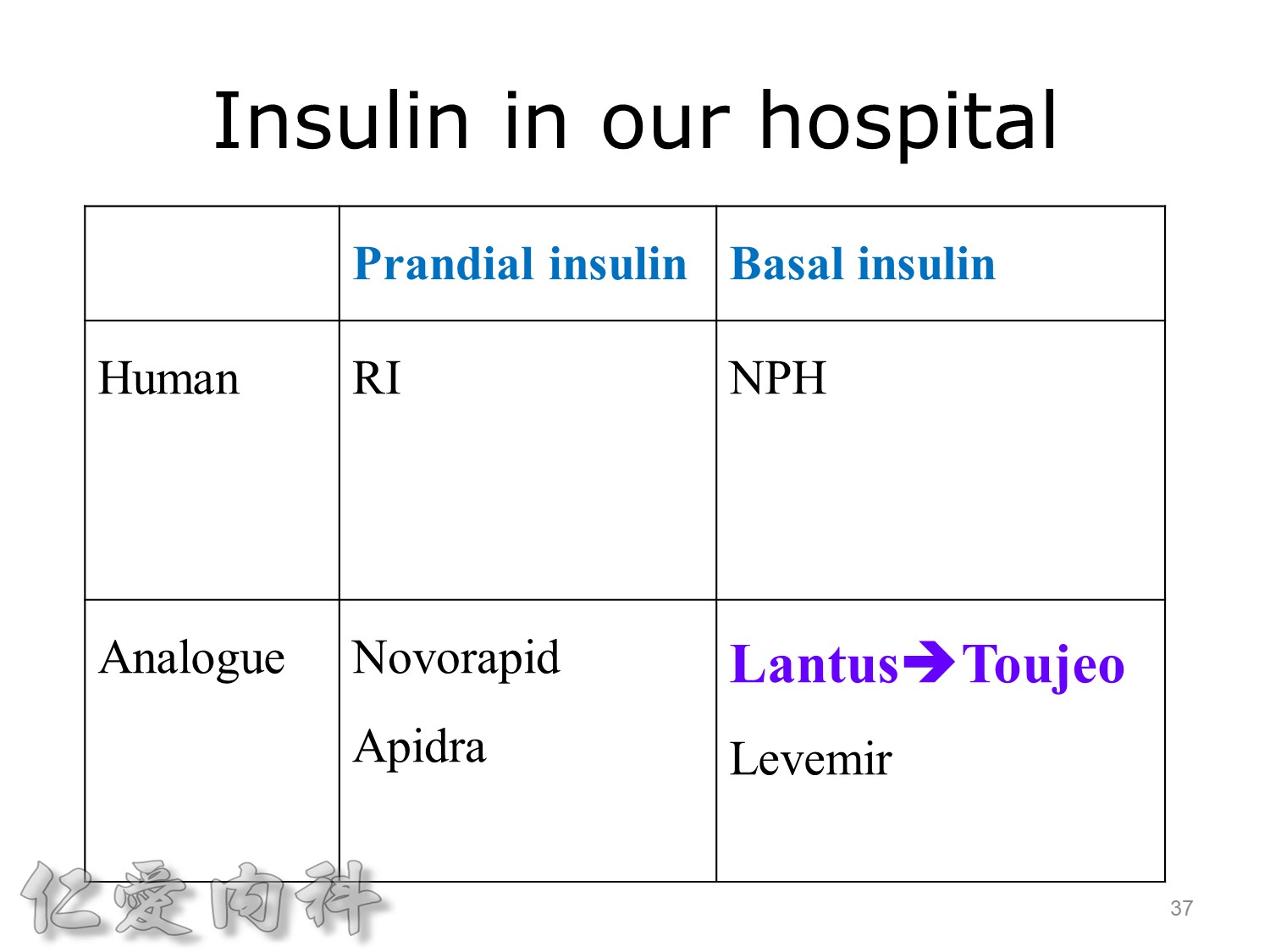
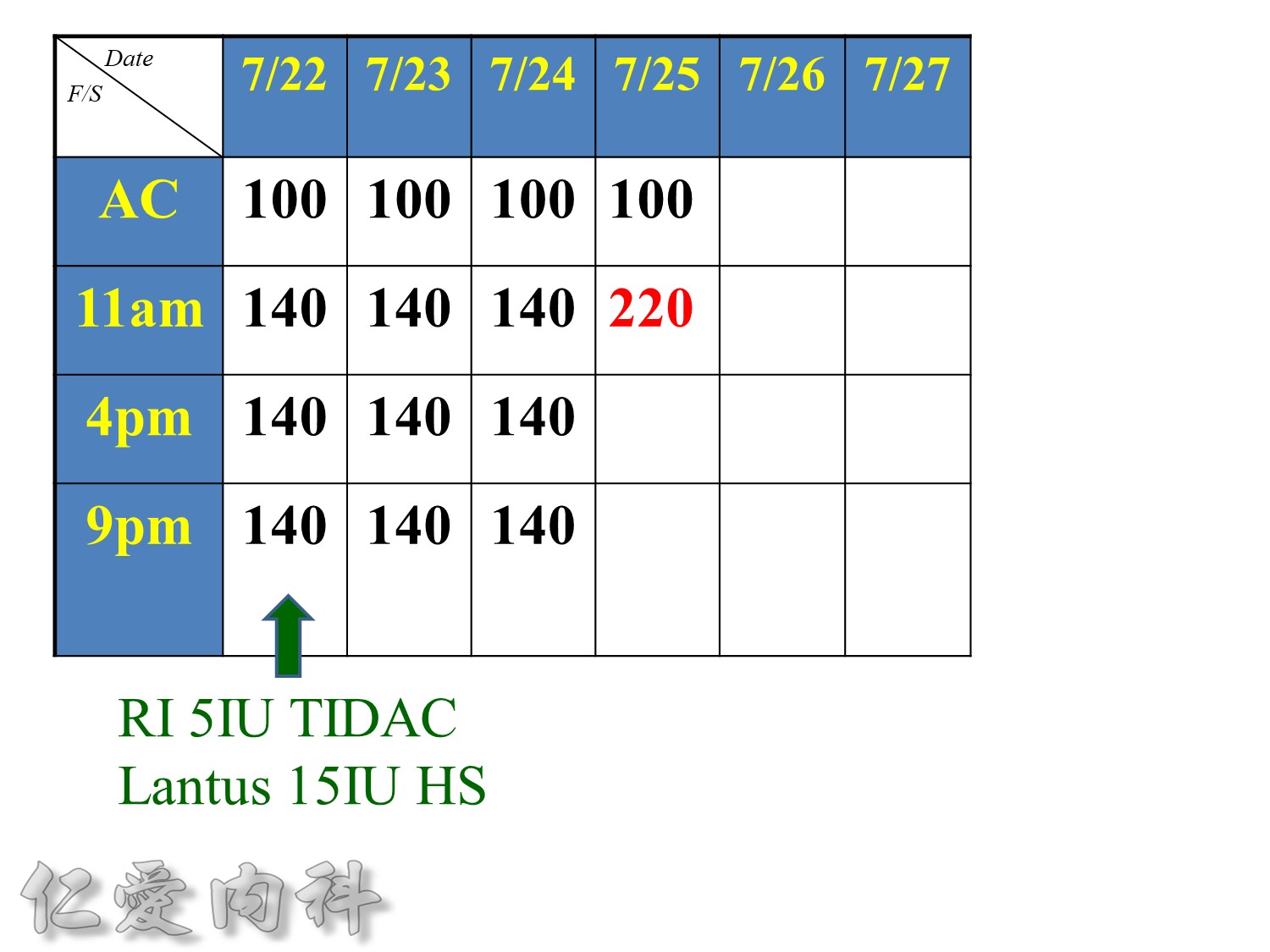
Basal insulin such as Toujeo 24 IU HS (照原來的劑量)==>打
但 Apidra ==> 不打
當INSULIN 功能只剩一半 ==> 也許在DM 發生多年後
Adapted from Buse JB et al. In Williams Textbook of Endocrinology. 10th ed. Philadelphia, Saunders, 2003:1427–1483;
Buchanan TA Clin Ther 2003;25(suppl B):B32–B46; Powers AC. In: Harrison’s Principles of Internal Medicine. 16th ed.
New York: McGraw-Hill, 2005:2152–2180; Rhodes CJ Science 2005;307:380–384.
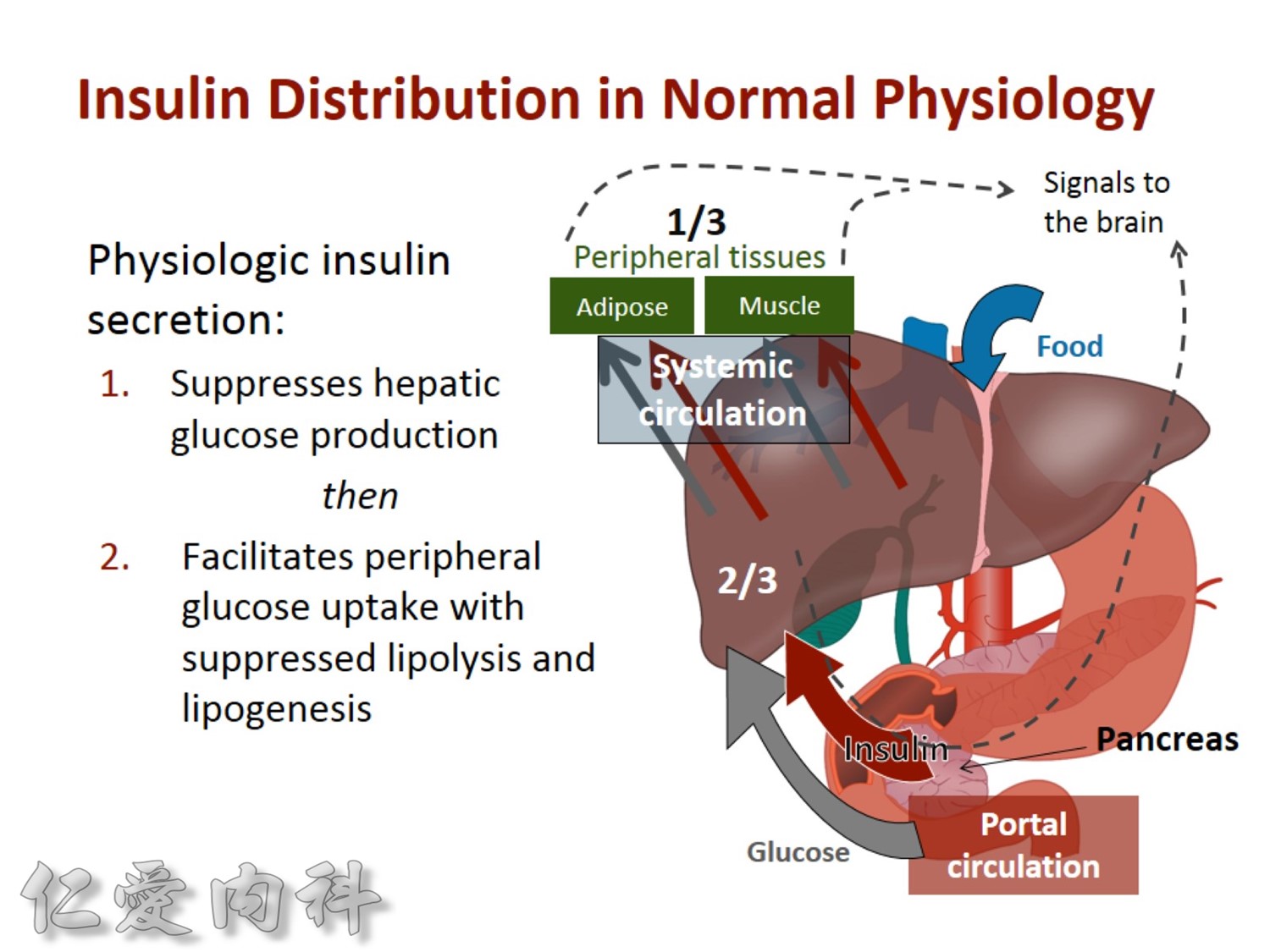
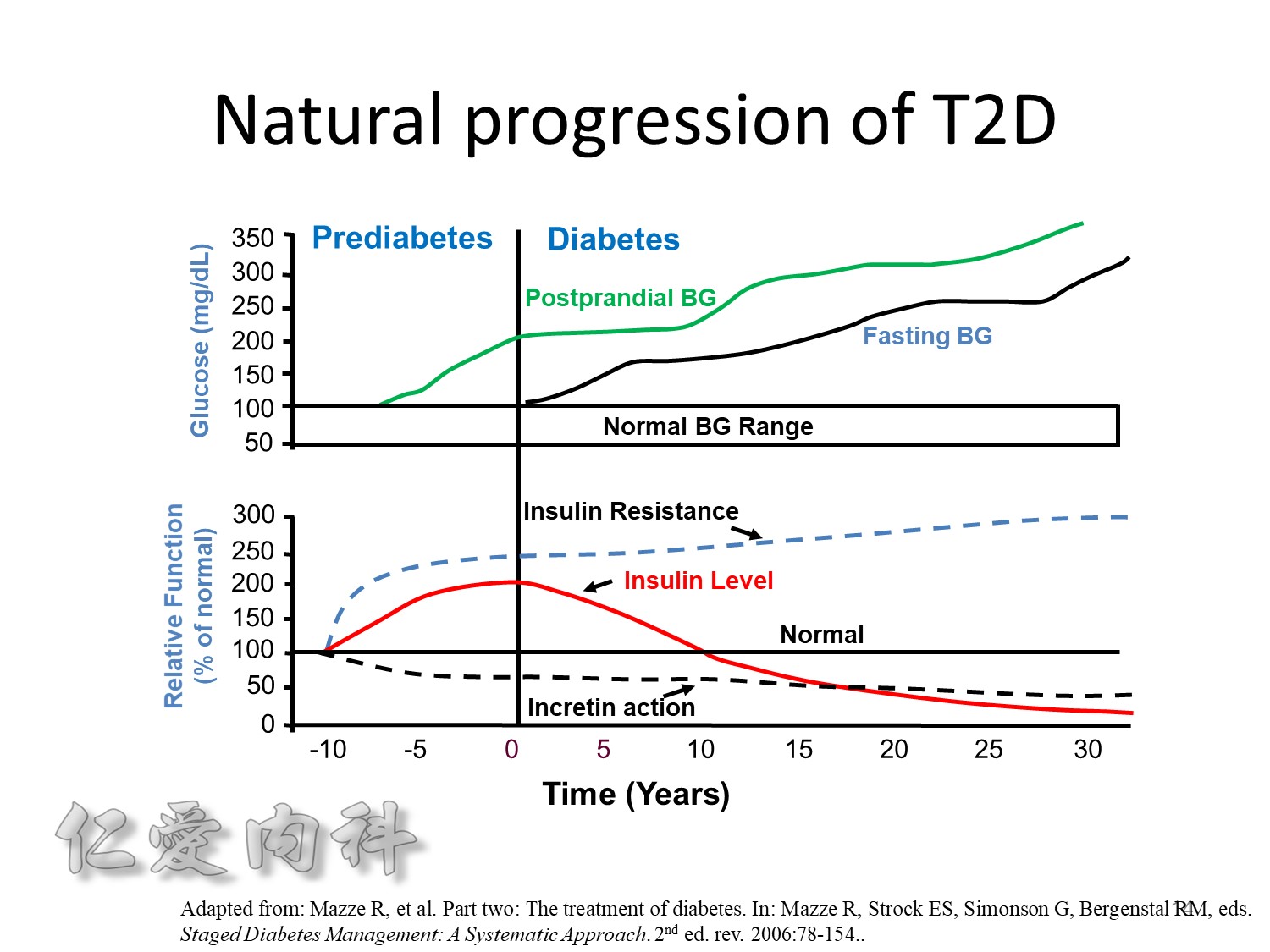
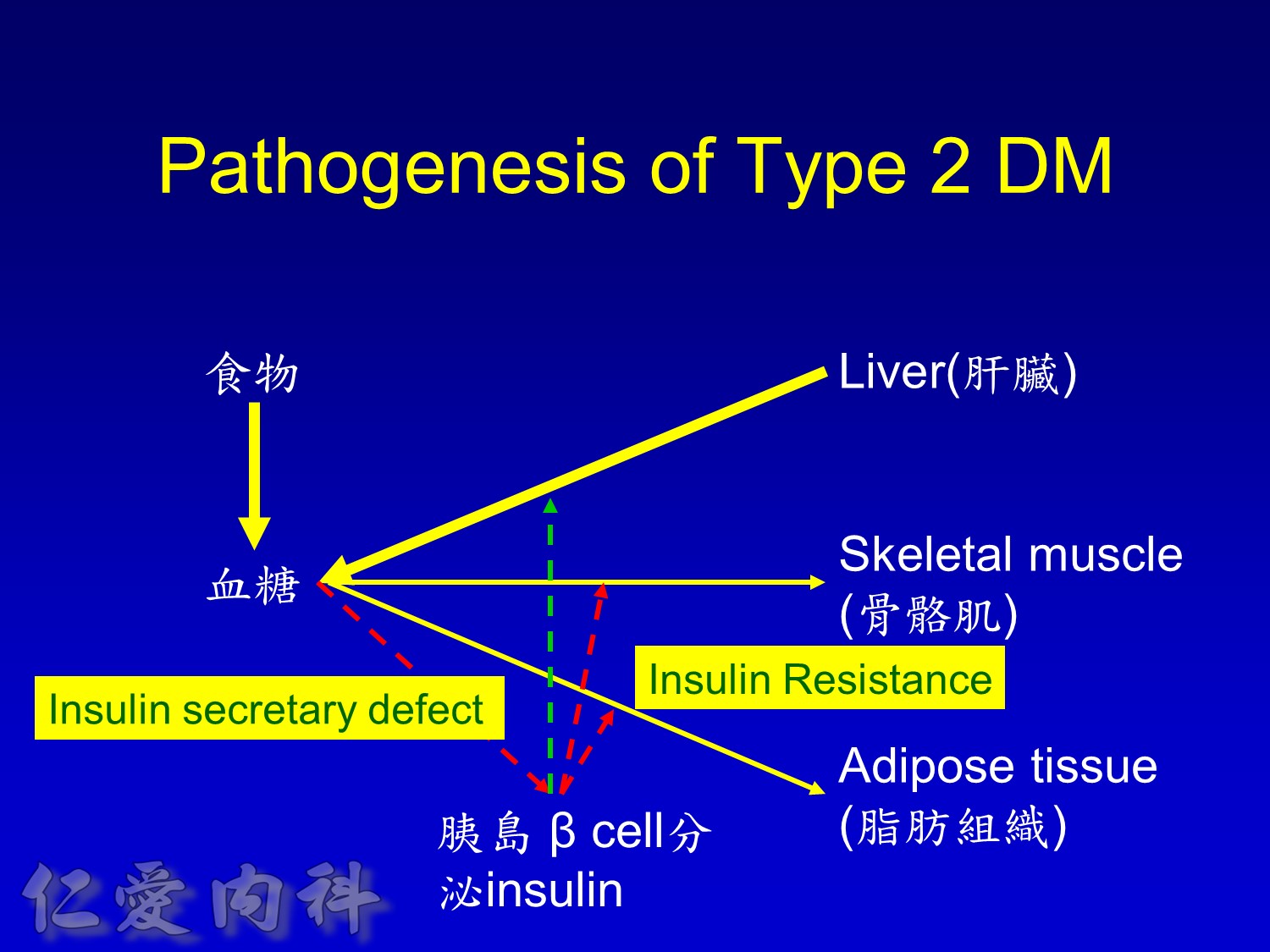
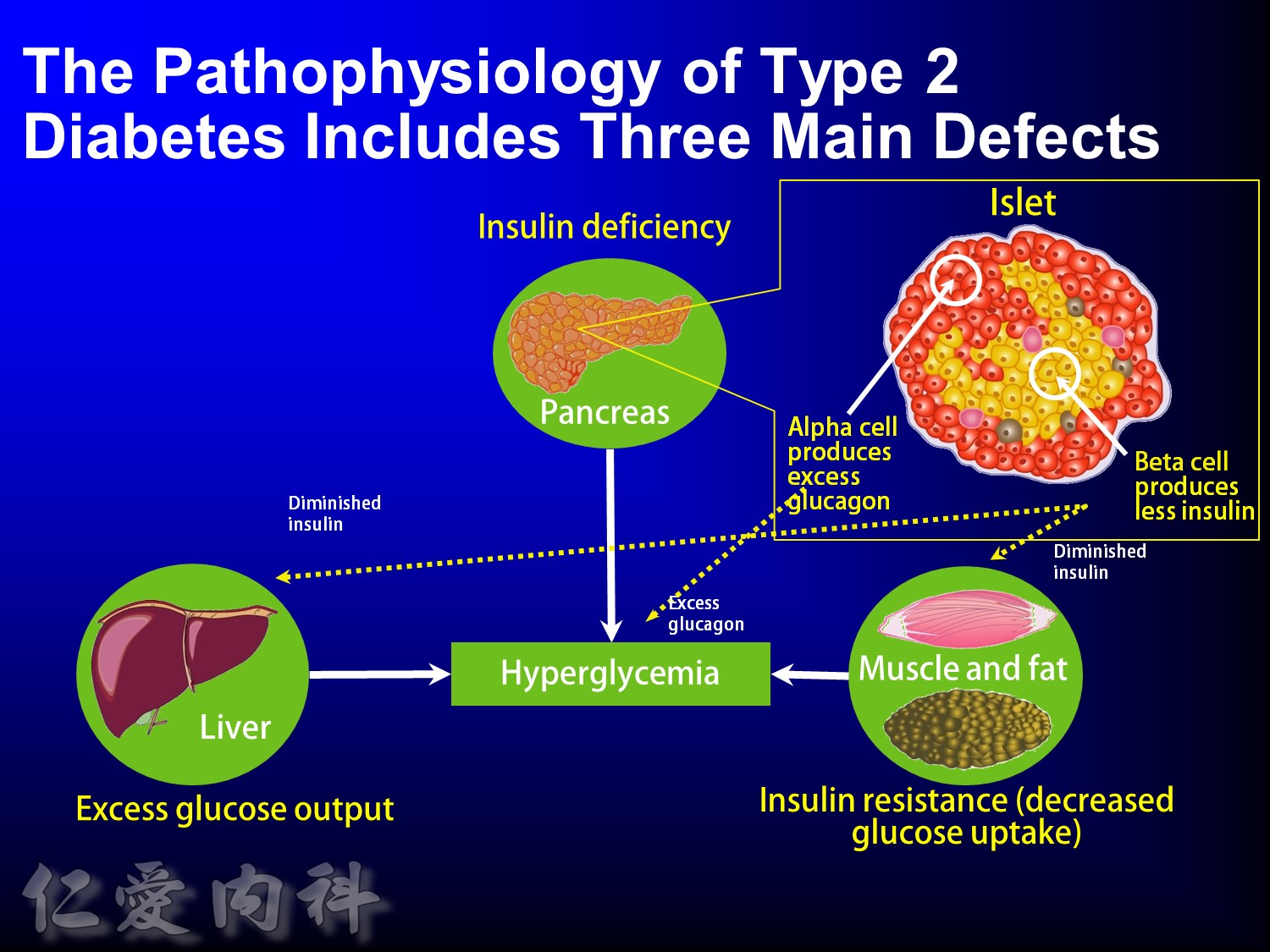
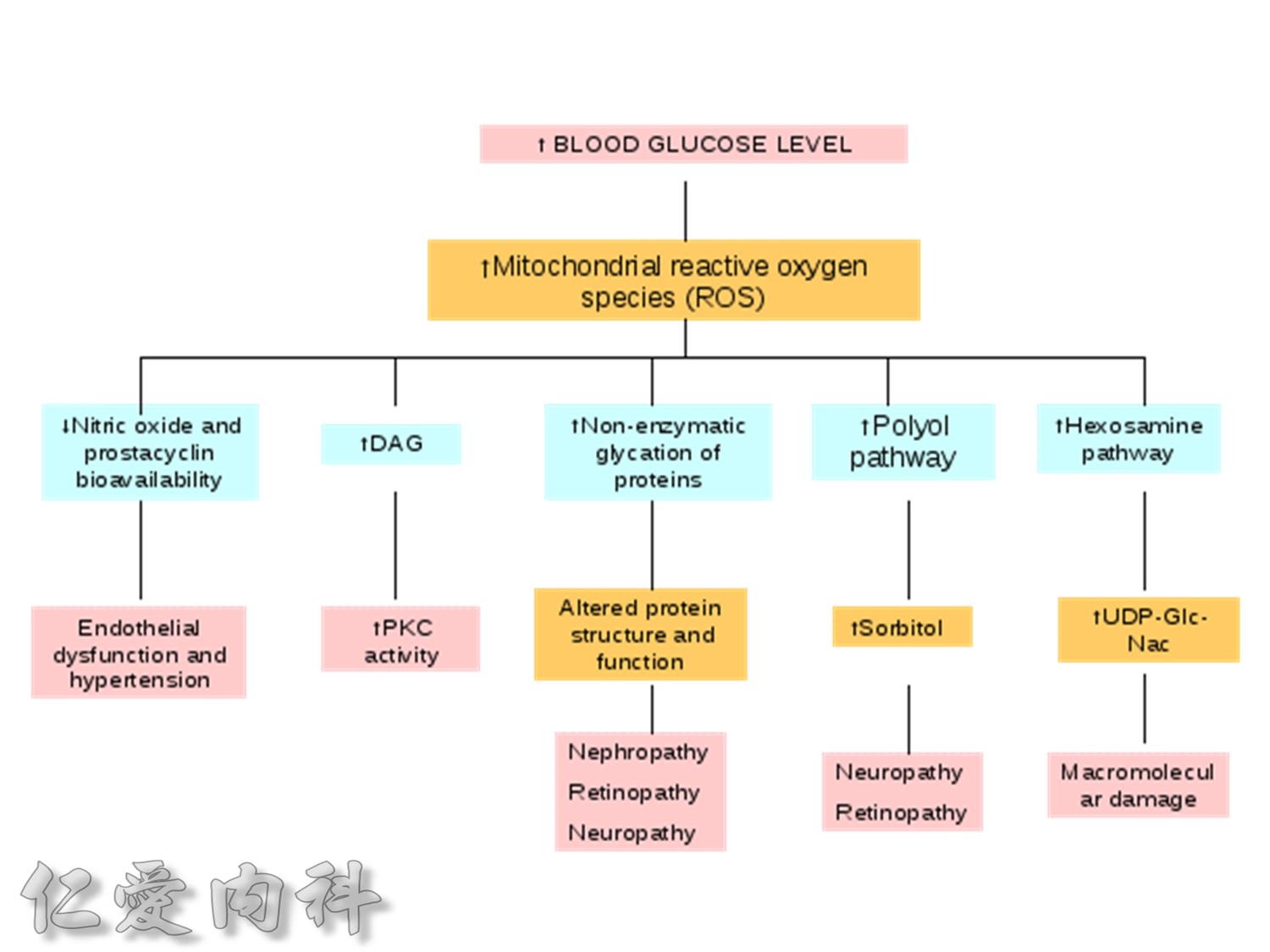
The pathophysiology of hyperglycemia in type 2 diabetes involves three main defects:
- insulin deficiency due to insufficient pancreatic insulin release;
- excess hepatic glucose output
- insulin resistance (decreased glucose uptake) in peripheral tissues (including muscle and fat) and the liver.
Two pancreatic islet cell defects contribute to this pathology:
- Beta cells produce insulin, which facilitates glucose entry into tissues. In type 2 diabetes mellitus, a decline in functional beta-cell mass causes insulin deficiency, which in turn contributes to hyperglycemia.
- Alpha cells produce glucagon.6 Elevated glucagon levels promote increased hepatic glucose output.
In type 2 diabetes mellitus, excess glucagon and diminished insulin secretion drive hepatic glucose output and contribute to hyperglycemia.
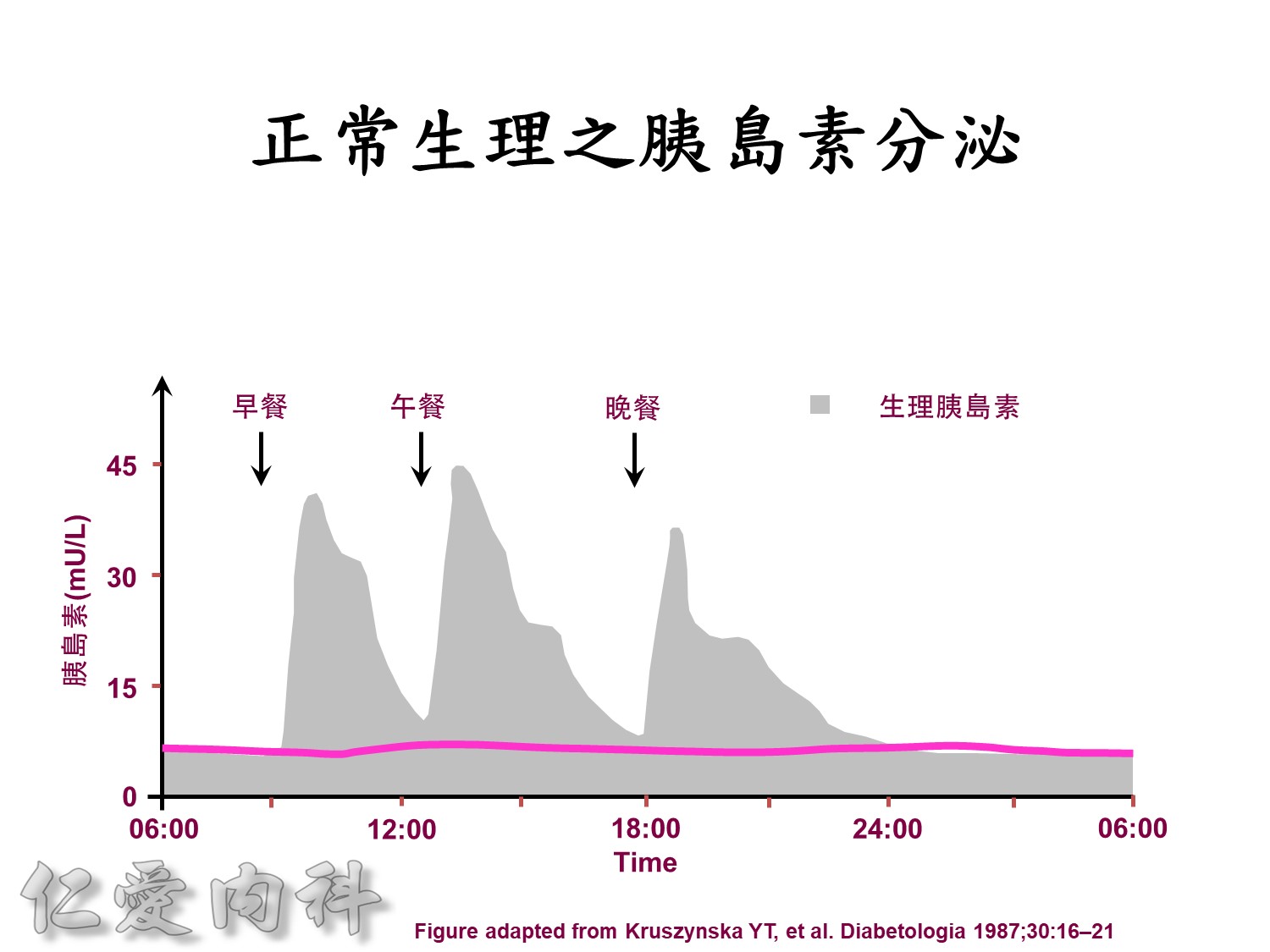
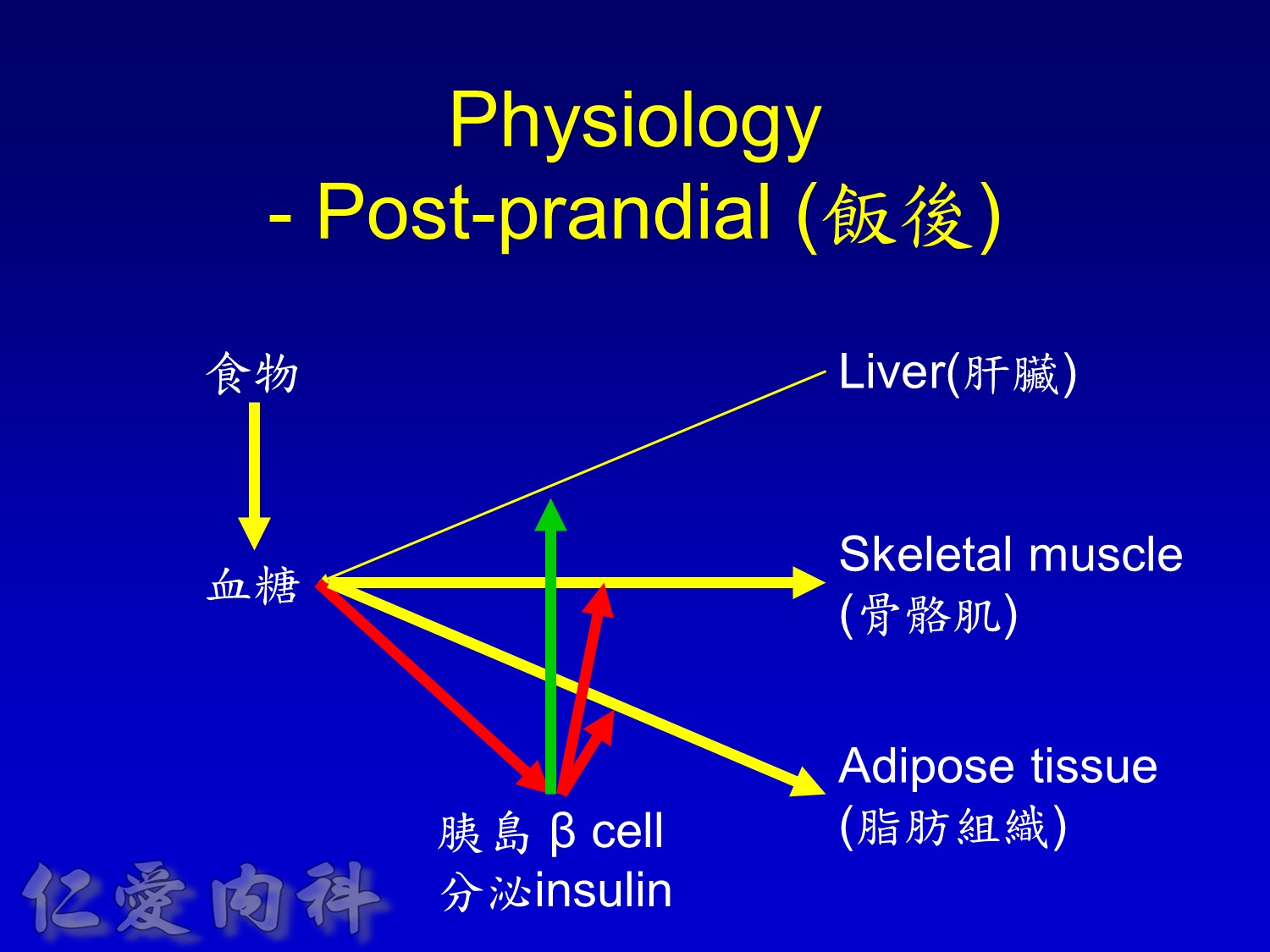
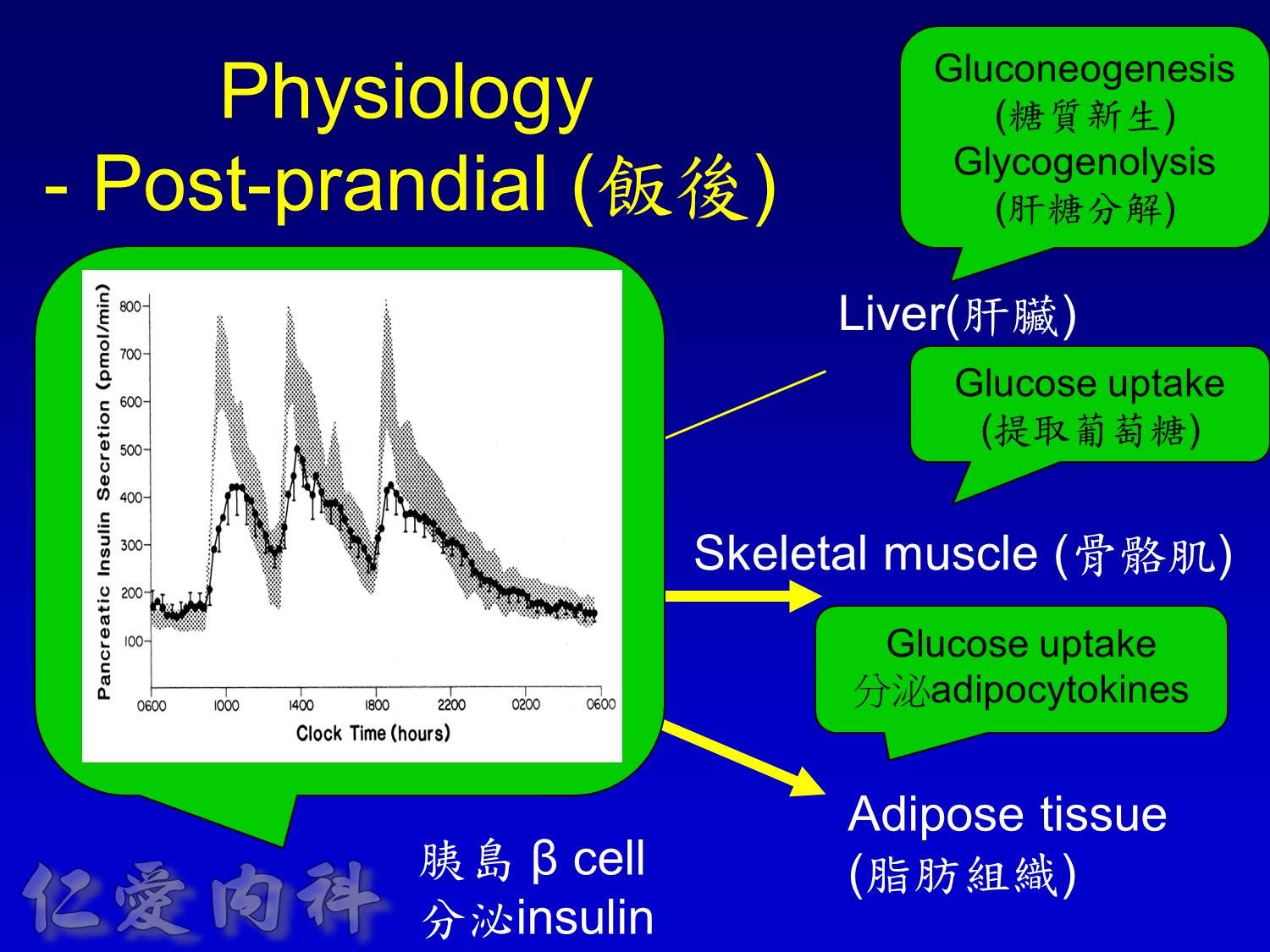
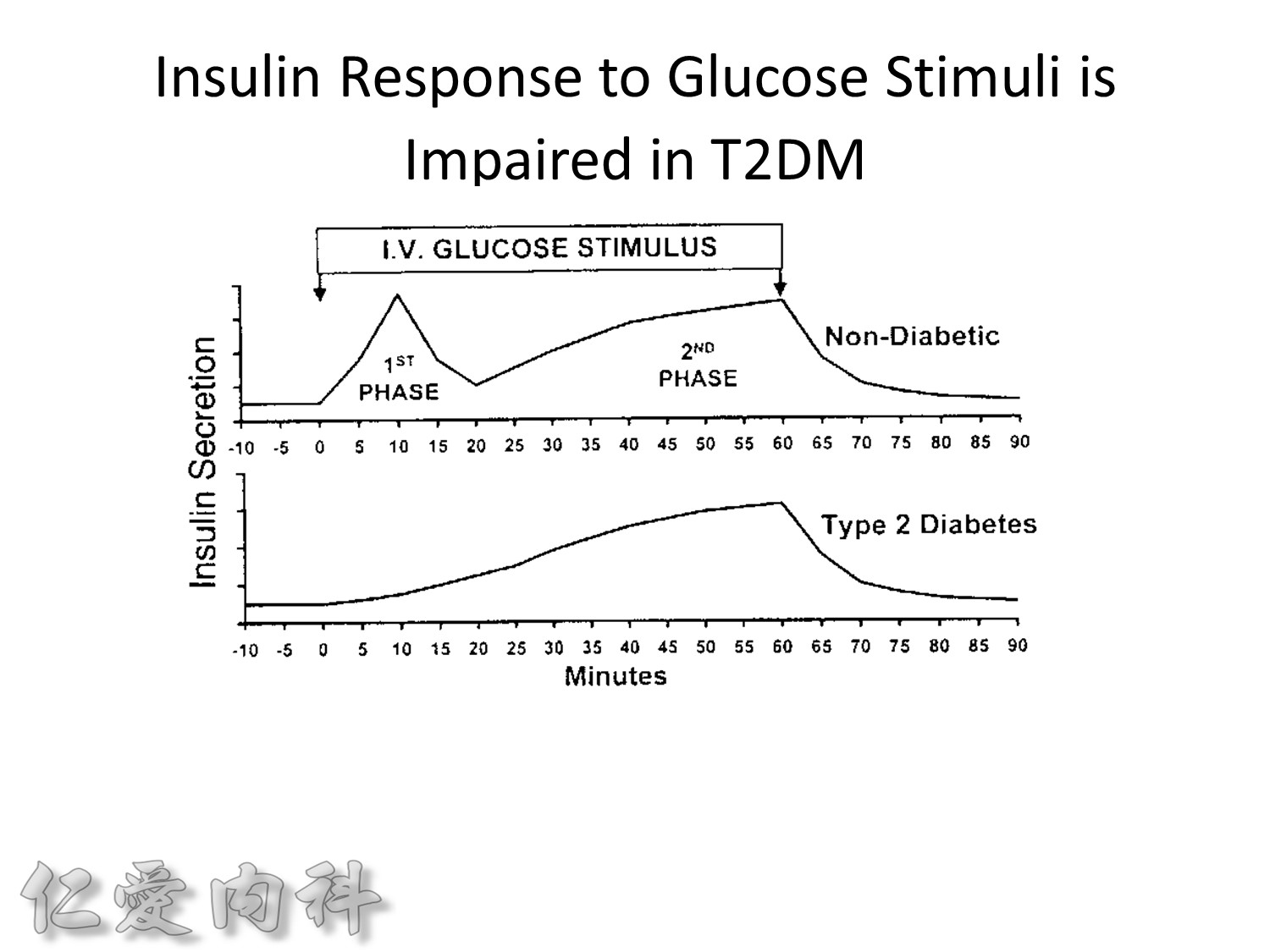
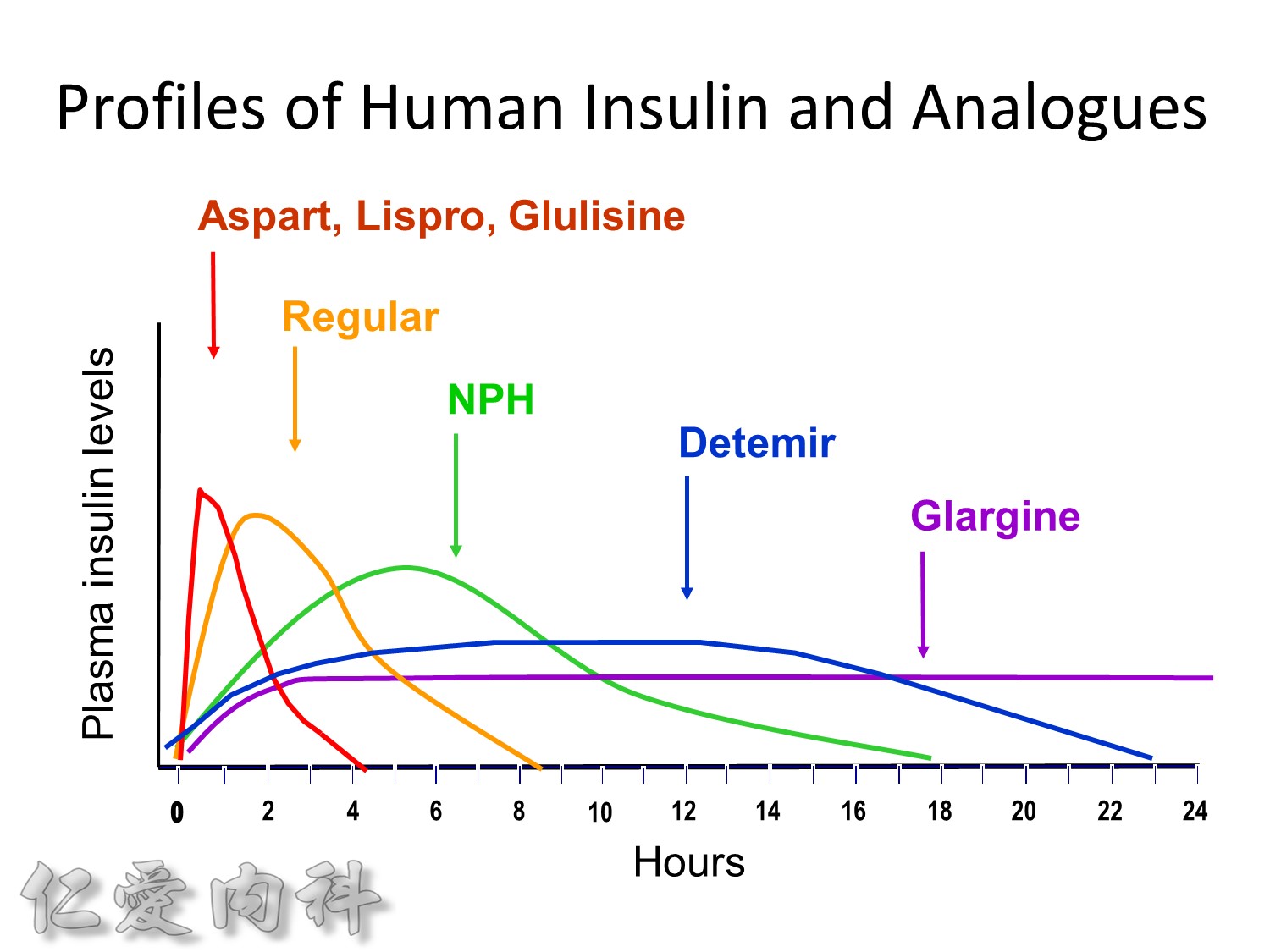
Insuin secretions divided into 2 phases
- First, rapid phase: stored insulin granules
- Second, slow phase: comprise of insulin synthesized de novo
NO DM==> 有1st phase (insulin會儲存胰島細胞的granules內) plus 2nd phase
(+)DM ==> 只有2nd phase
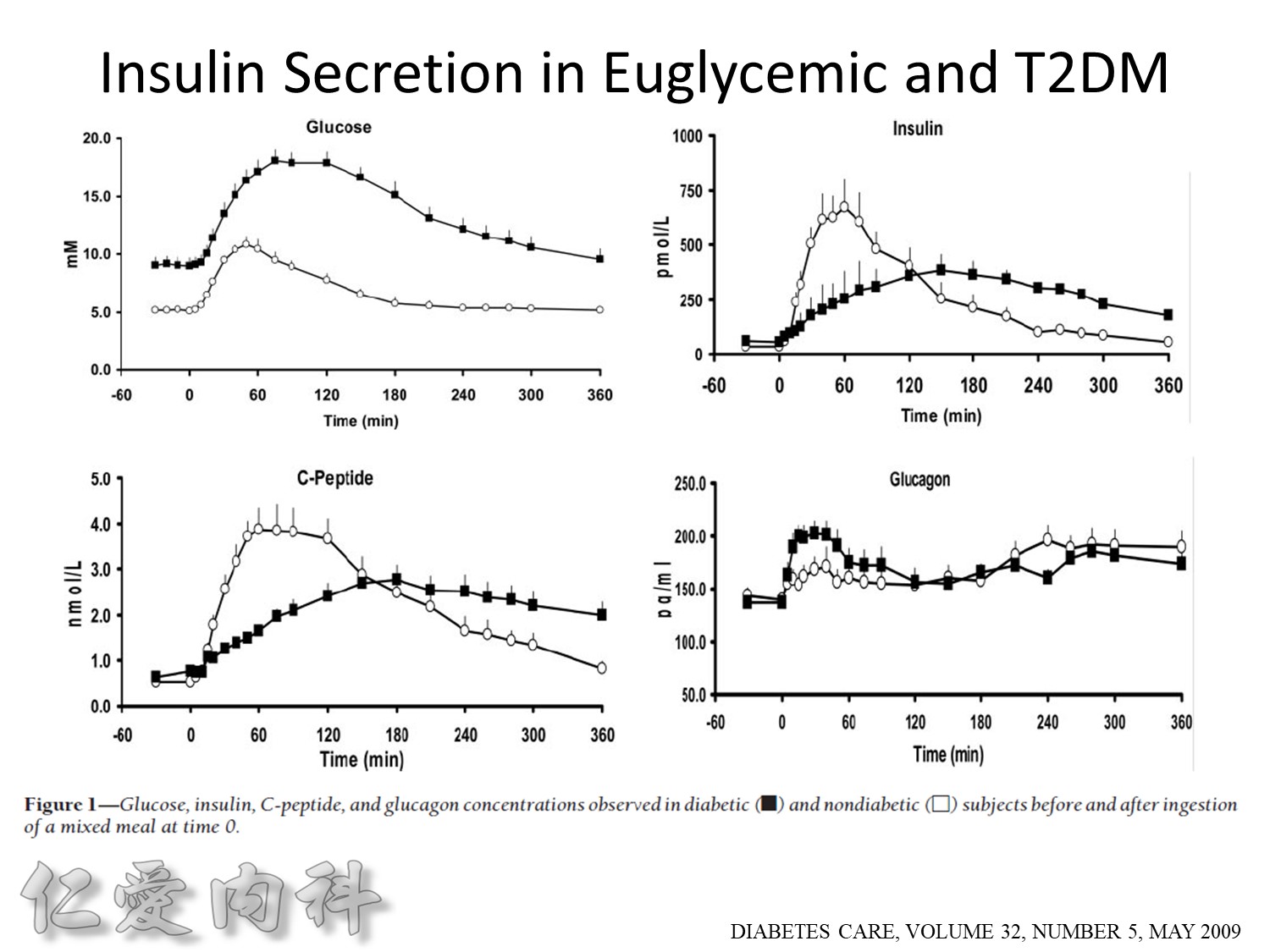
Euglycemic subjects
- Plasma insulin peaks at 1 hour.
- Return to baseline at 4 hours.
DM subjects
- Lower peak
- Delayed insulin secretion and overshoot
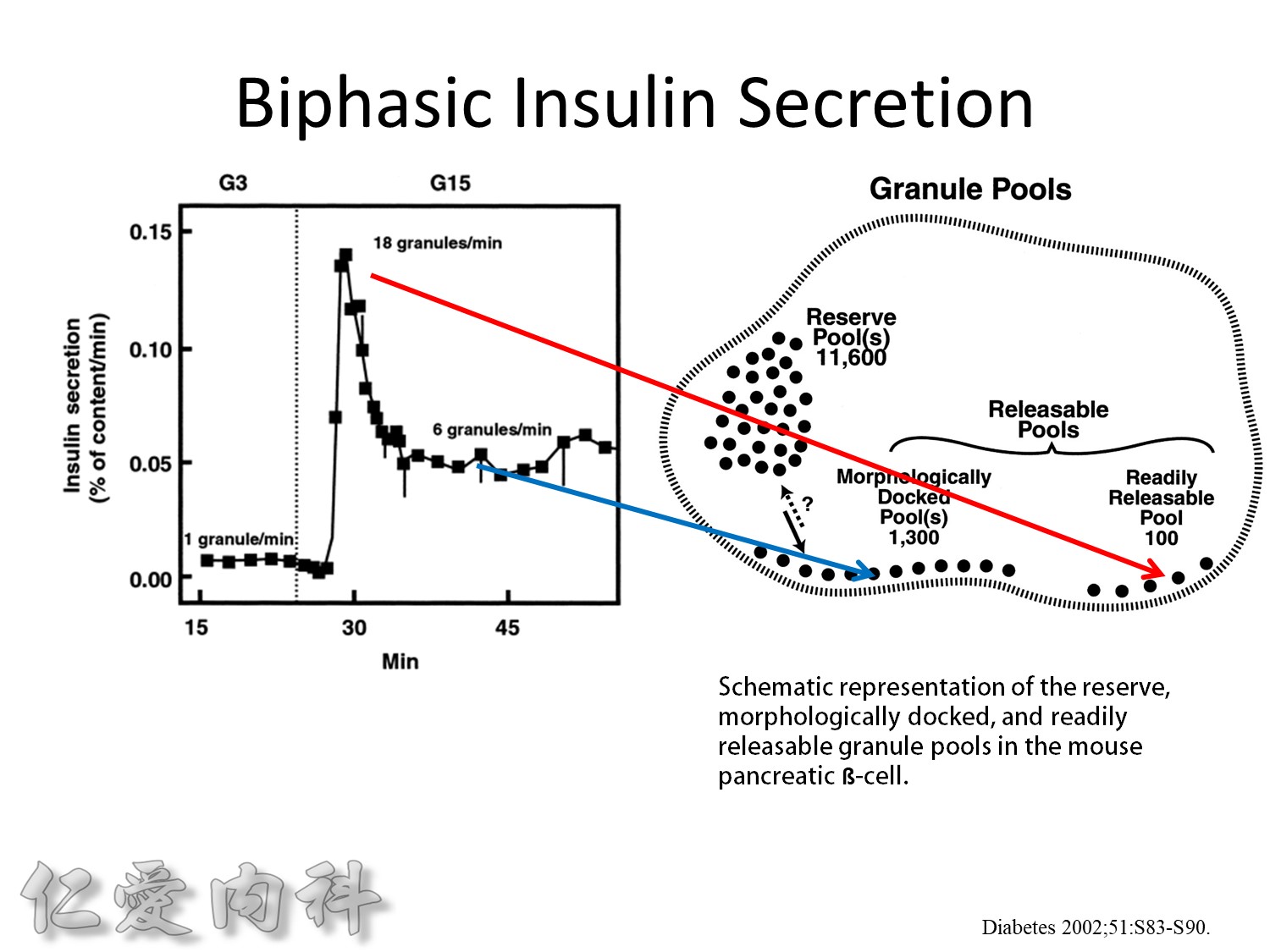
The biphasic insulin secretory response to 16.7 mmol/l glucose by mouse islets. The results are expressed as percent of total insulin content released per minute. Also shown are the number of granules released per ß-cell per minute under basal conditions, at the peak of the first phase, and during the second phase.
This is from the cultured mouse islets
前鋒 主力
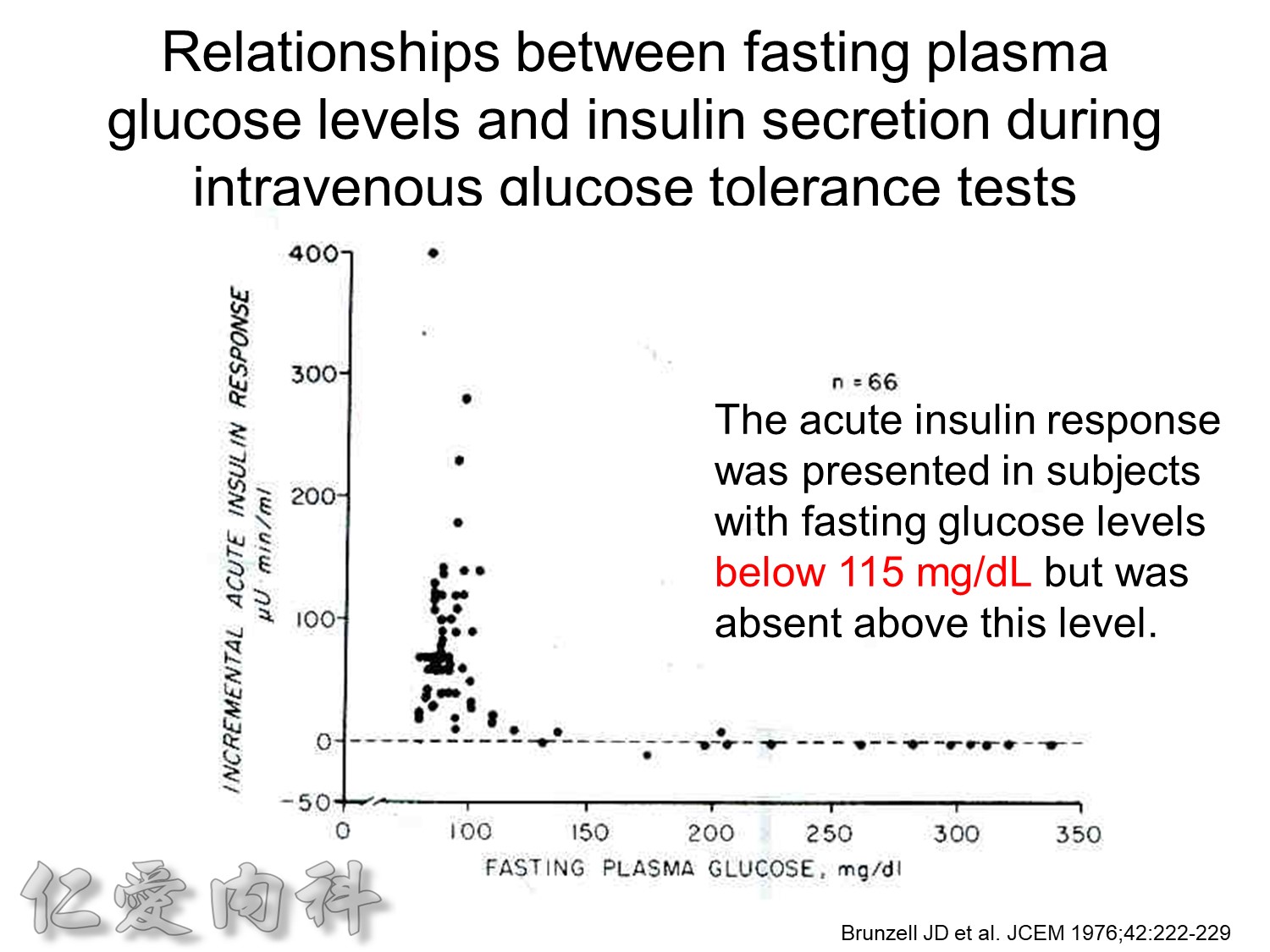
Ac sugar 約在115 mg/dL 才有2nd phase!

大約平均1 IU insulin 可降20-30mg/dL 的血糖(有時可降多達50mg/dL左右)

以前insulin 是從動物提煉的容易過敏!!


希望透過今天主角下一代胰島素 Toujeo的介紹,可以讓在座每一位醫師,幫助您的病患接受使用下一代胰島素 Toujeo,讓血糖控制可以達標,無須妥協低血糖與治療目標的窘境。
Toujeo 劑量的起手勢 ==>0.2 ==> 0.25 IU/kg
Decrease gluconeogenesis plus exercise ==> better blood sugar control
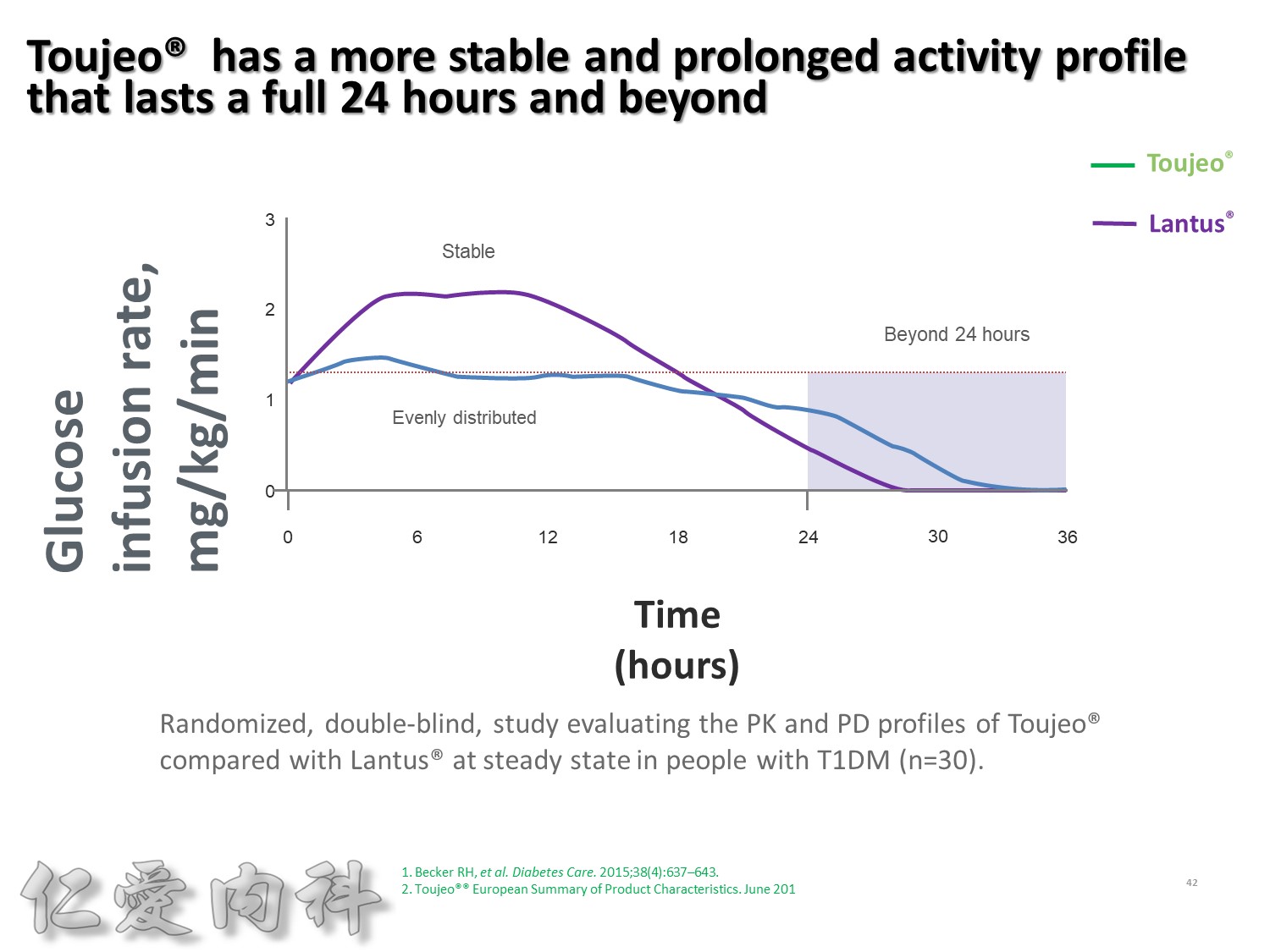
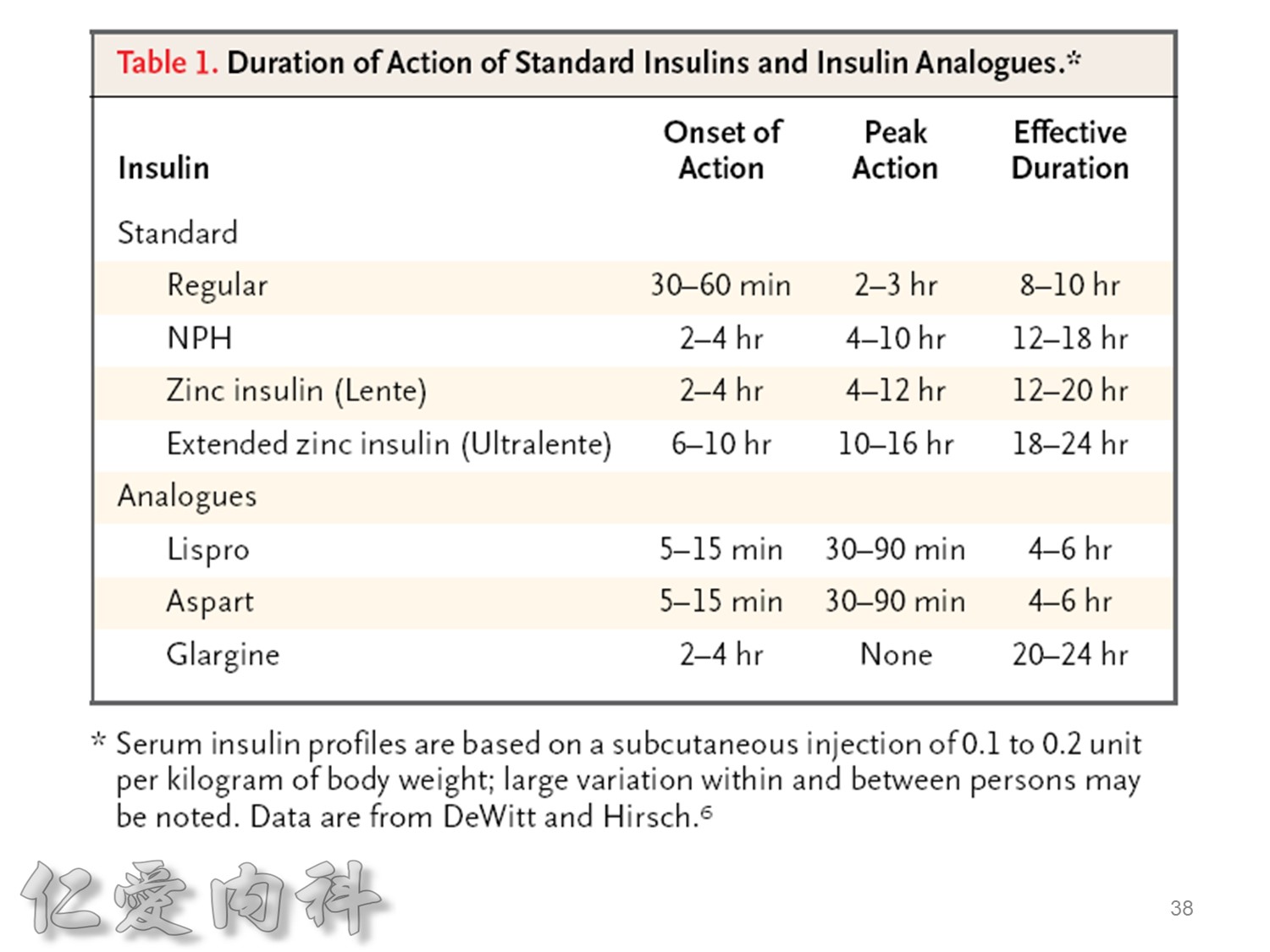
Lantus作用時間如同醫師知道的, 是24小時。
Toujeo則是Beyond 24小時 (Beyond=超過), 完整的 Cover 24小時, 而釋放時間更加的平穩。
此圖是Toujeo與Lantus各用0.4 IU/kg在 T1DM 病人身上觀察藥效學的差異。
可以看到在24小時內, Toujeo 的 GIR 比Lantus平穩。
顯示Toujeo擁有更平穩且作用持續超過24小時的血糖控制能力。
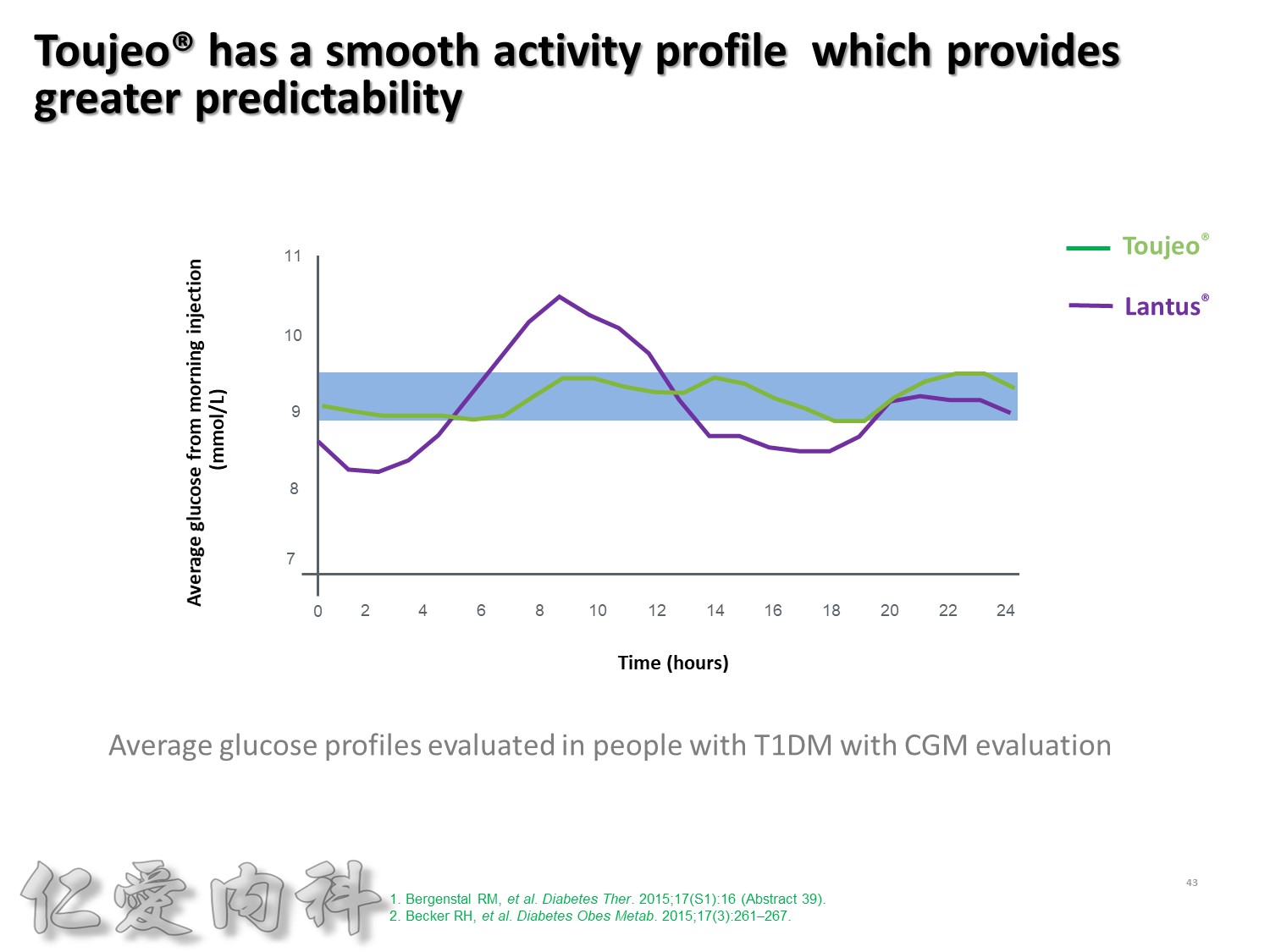
這樣緻密的結果, 使得Toujeo 24小時的釋放狀況更加的平穩, 如果你覺得Lantus的平穩程度是賓士的話, Toujeo就是賓利。
大家可以看到這樣的血糖波動真的是非常非常的平穩。
此圖是一項利用CGM方式, 觀察T1DM以每天早上或睡前注射一次Toujeo和Lantus, 兩者控制血糖能力的試驗。
這張圖是兩者在早上注射的結果, 顯示Toujeo擁有更平穩的血糖控制能力。

Basal insulin QD or HS sc , better blood sugar control
==> Lantus HS sc 較好, Levemir 若 QD sc ==> 較差
但新一代的胰島素例如 Toujeo –>早上或晚上打沒有差別!
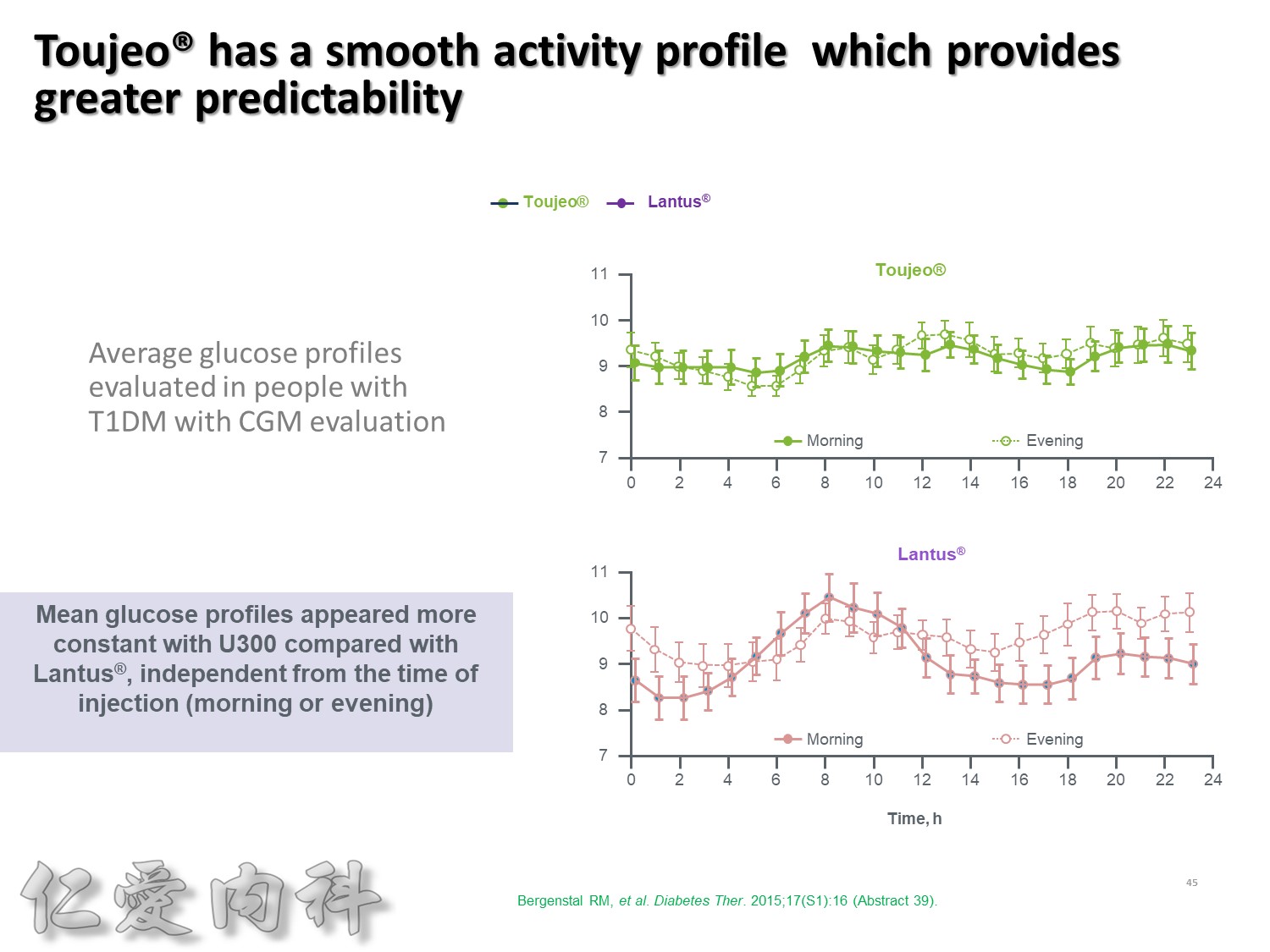
更重要的, 不管是早上施打, 及晚上施打, Toujeo呈現的波動度都是相當的平穩。
後面會提到Toujeo在亞洲人的實驗, 夜間低血糖比Lantus減少了一半。
所以這就是為什麼, Toujeo是新一代的胰島素。

先控制AC sugar 較好, 然後等到1st phase of insulin secretion restore then再控制PC sugar。
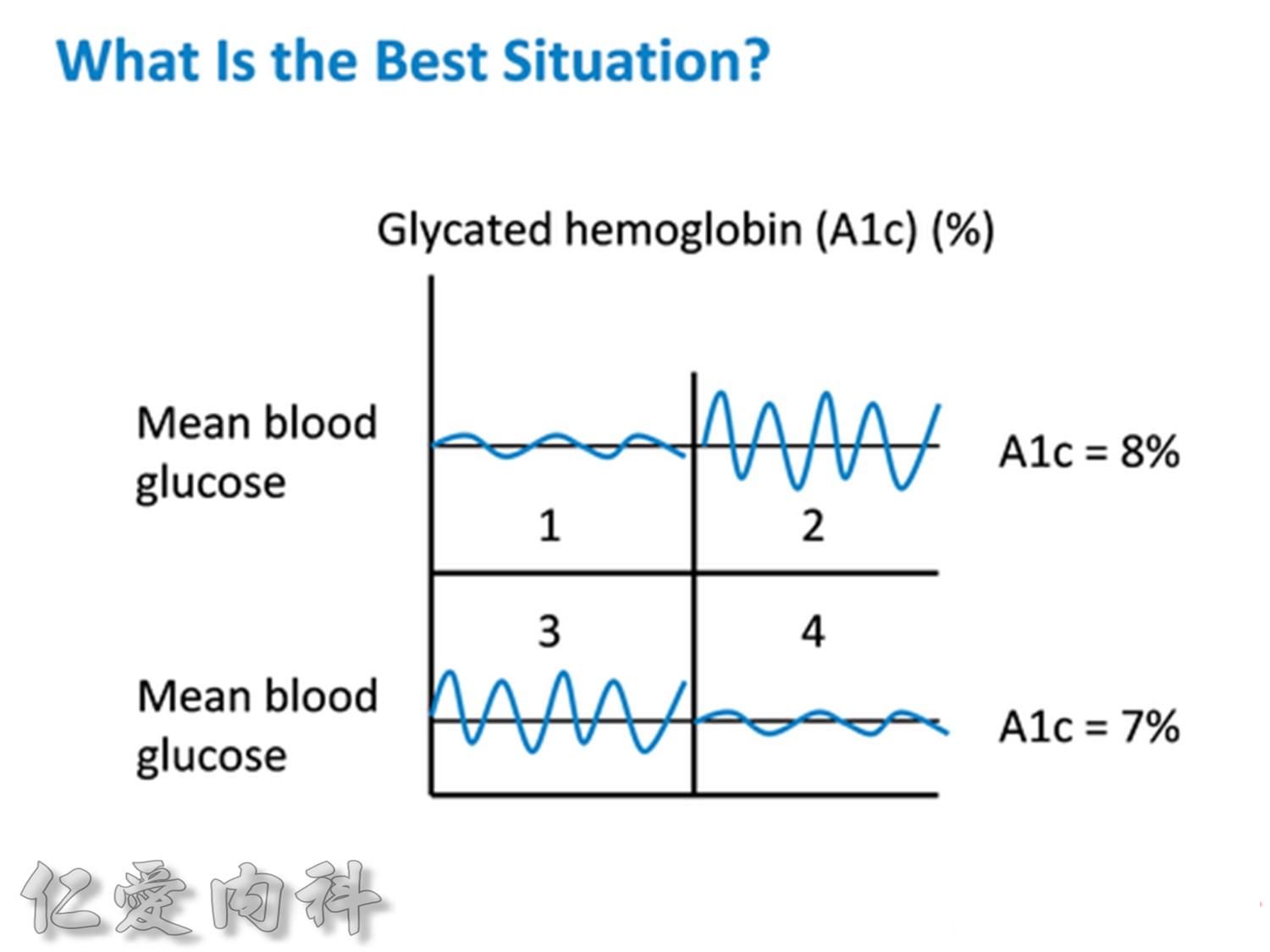
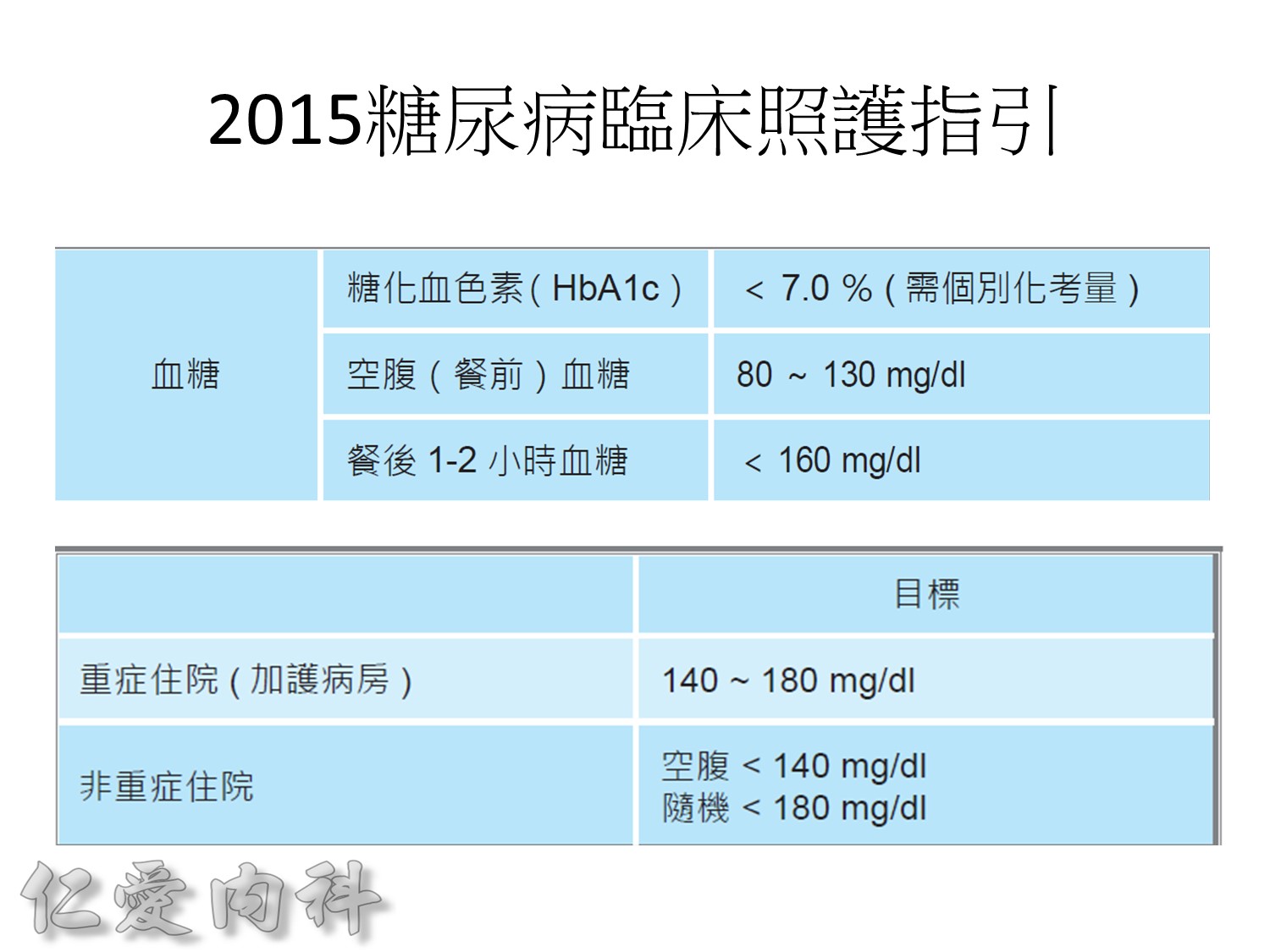
Usually, we should control our HbA1c to be between 6.5 and 7.0%.
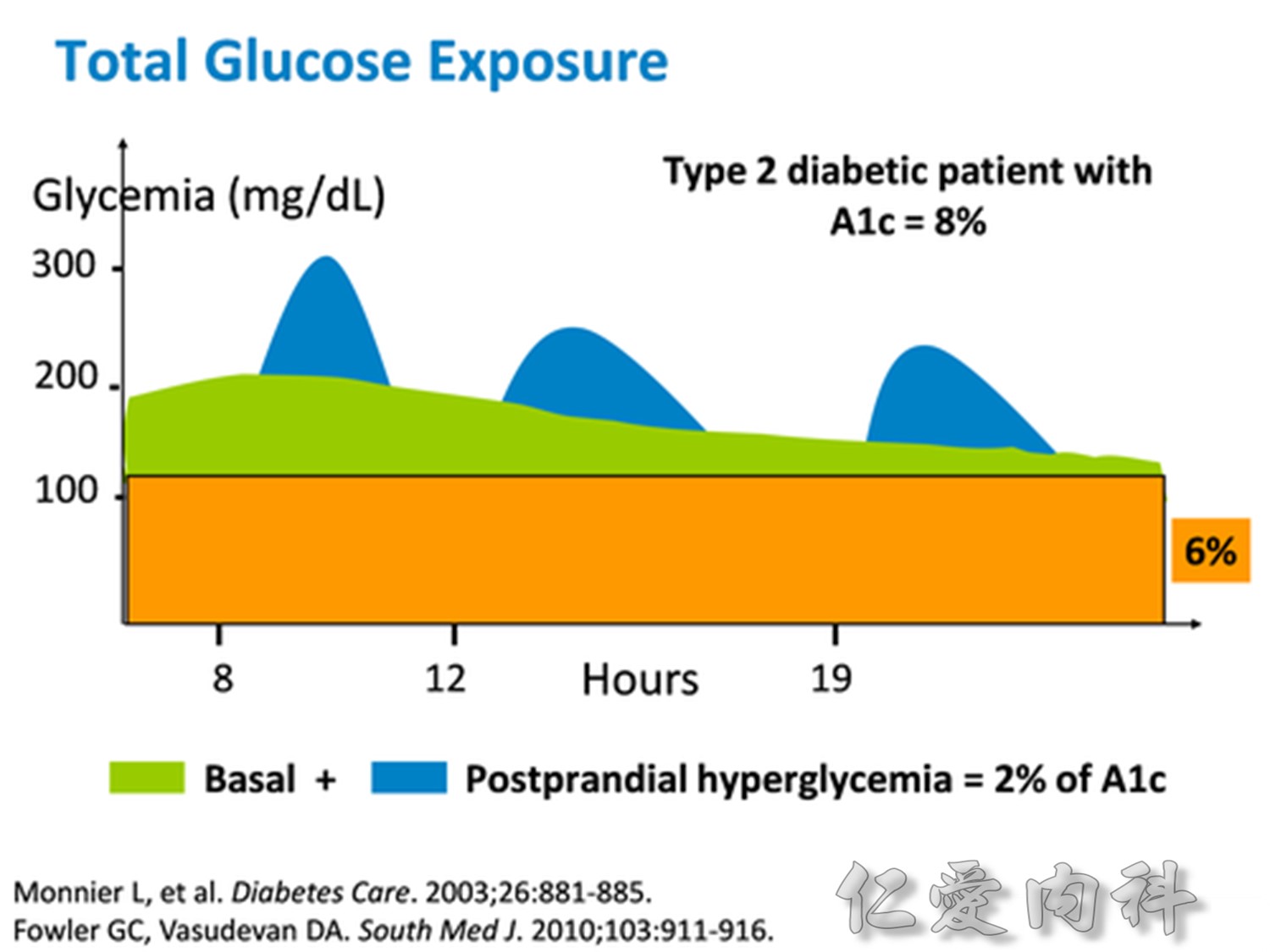
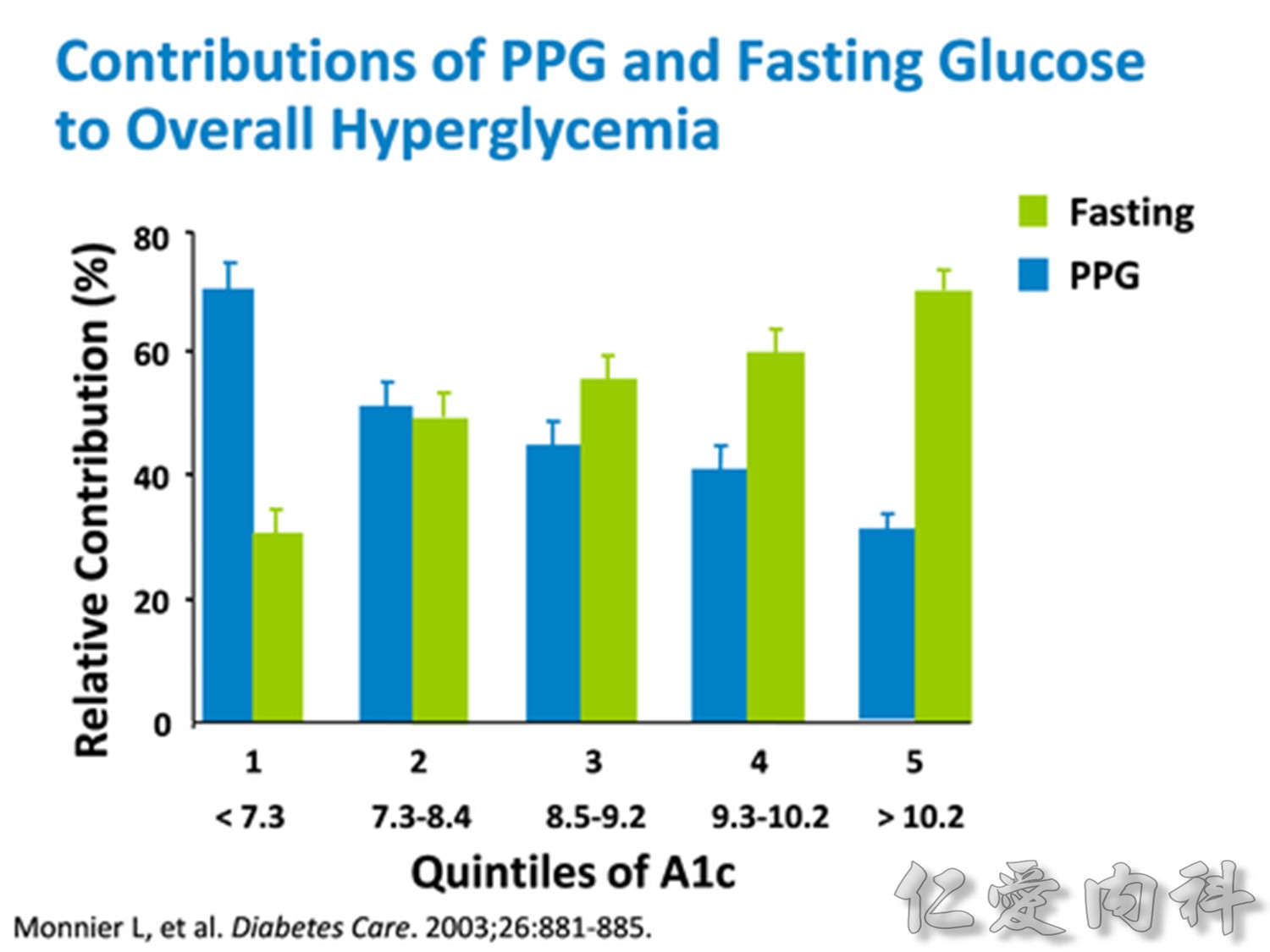
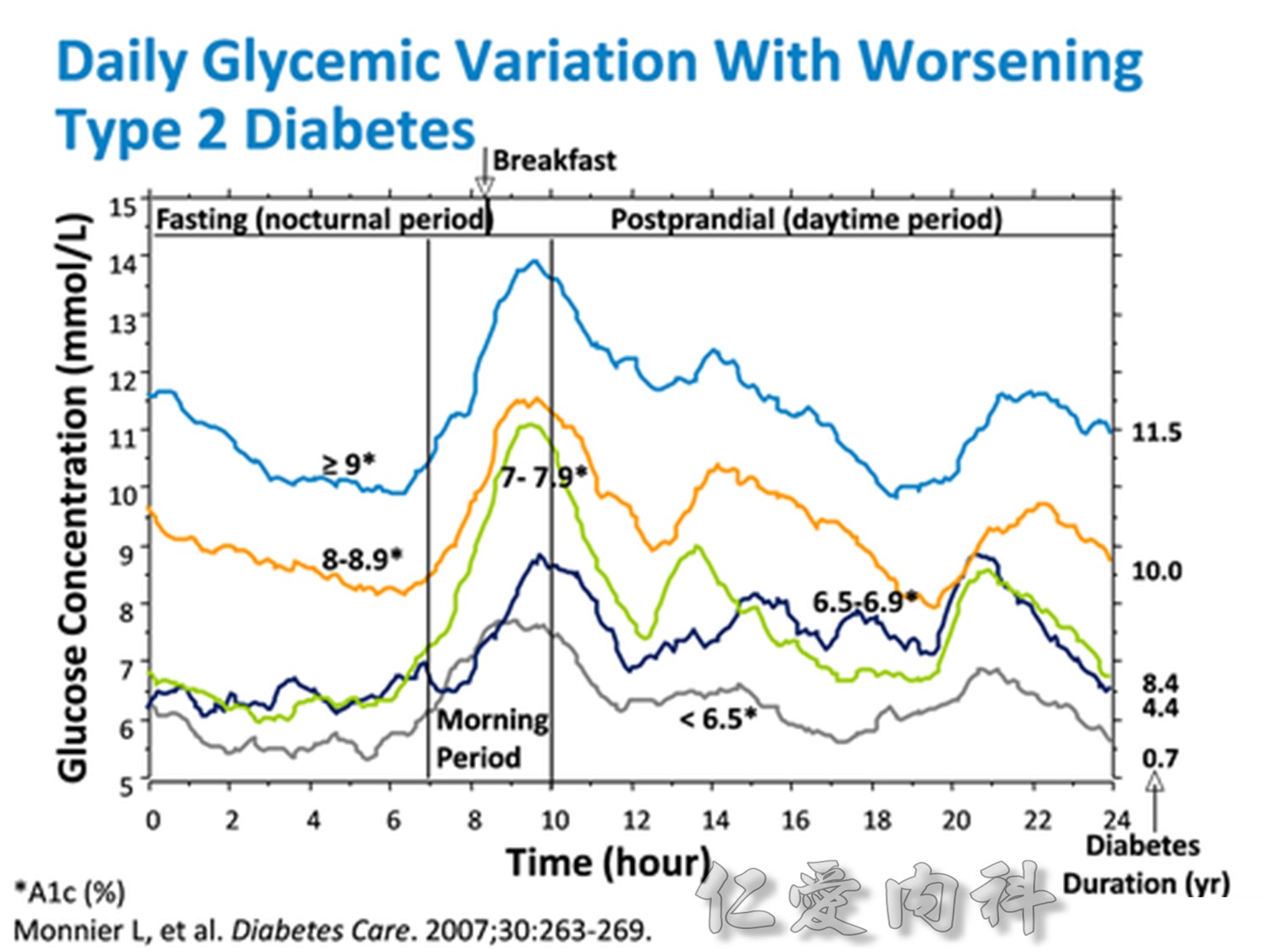
Comparison of 24-hour glucose levels in control subjects vs patients with diabetes (p<0.001).
Adapted from Hirsch I, et al. Clin Diabetes 2005;23:78–86.
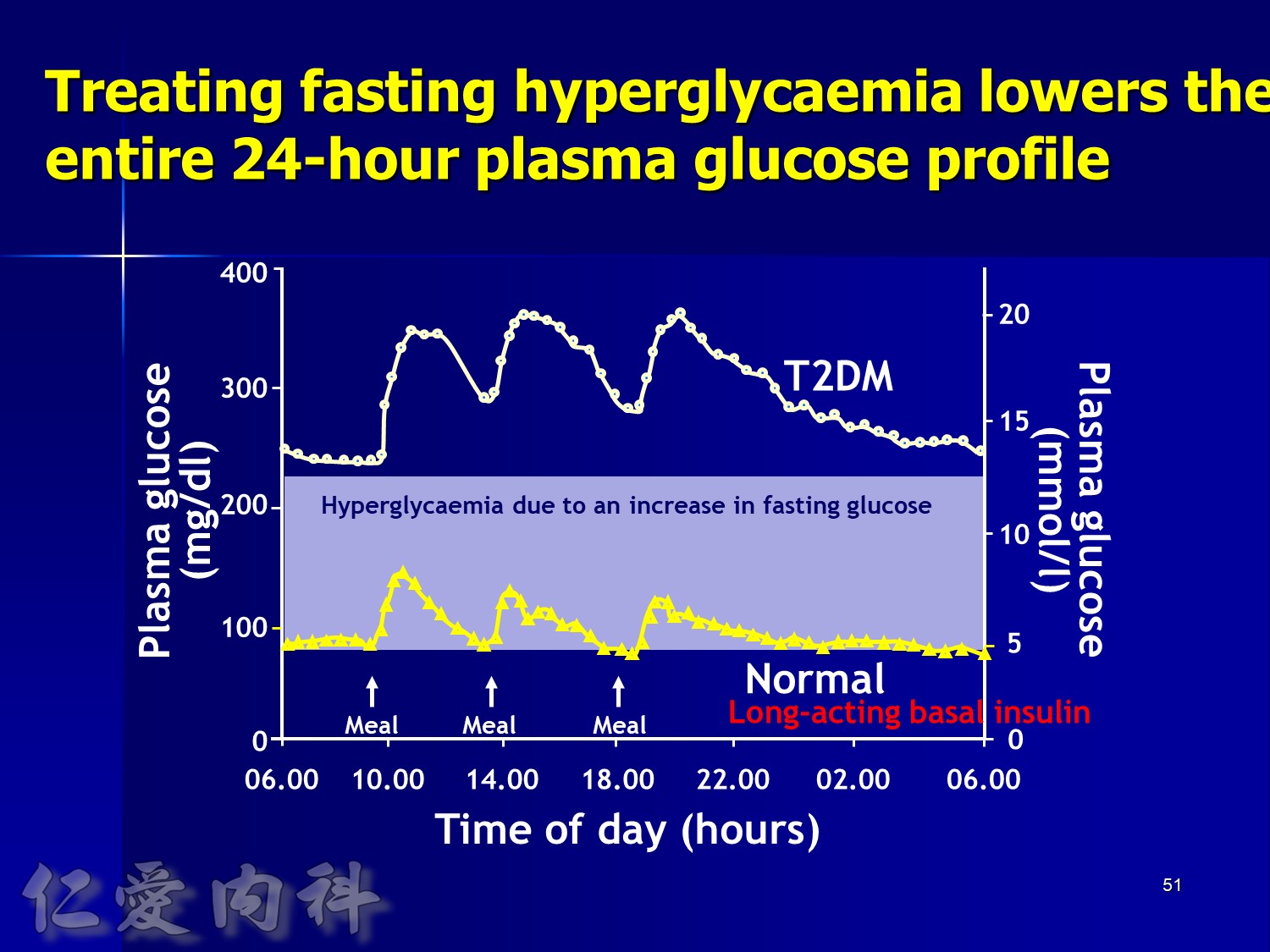
In patients with T2DM, excess exposure to hyperglycaemia is caused by an increase in fasting plasma glucose concentrations.
Specifically targeting fasting hyperglycaemia will therefore lower the entire 24-hour plasma glucose profile and should be a primary aim of therapy.
Hirsch I, et al. Clin Diabetes 2005;23:78–86.




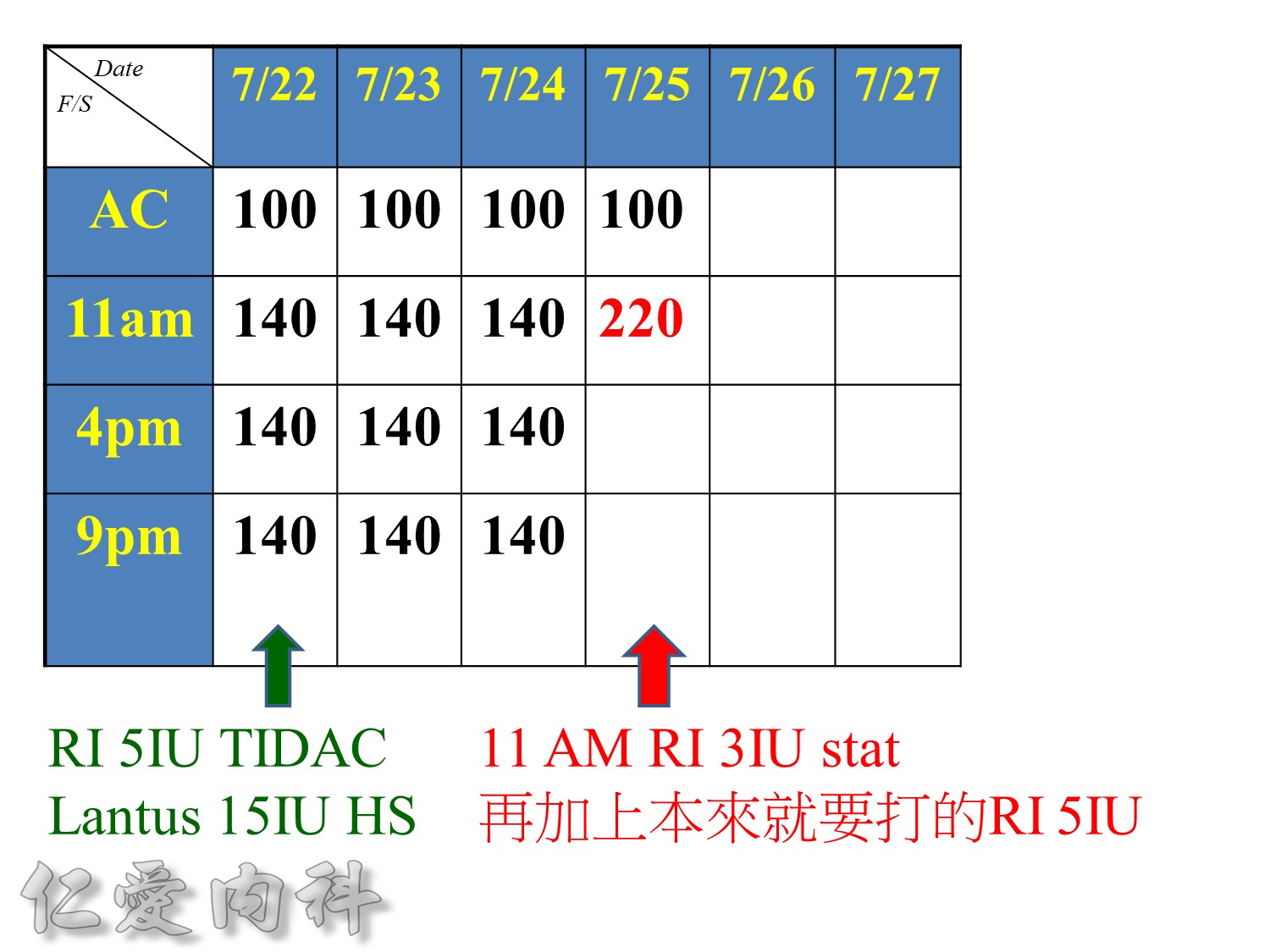
請注意!
考慮病人的現在血糖, 使用藥物, 肥胖程度及insulin resistance程度來調整起始劑量
==> 避免低血糖

請注意!
若擔心低血糖,可以從Daily dose 0.5 IU/kg/day 給起

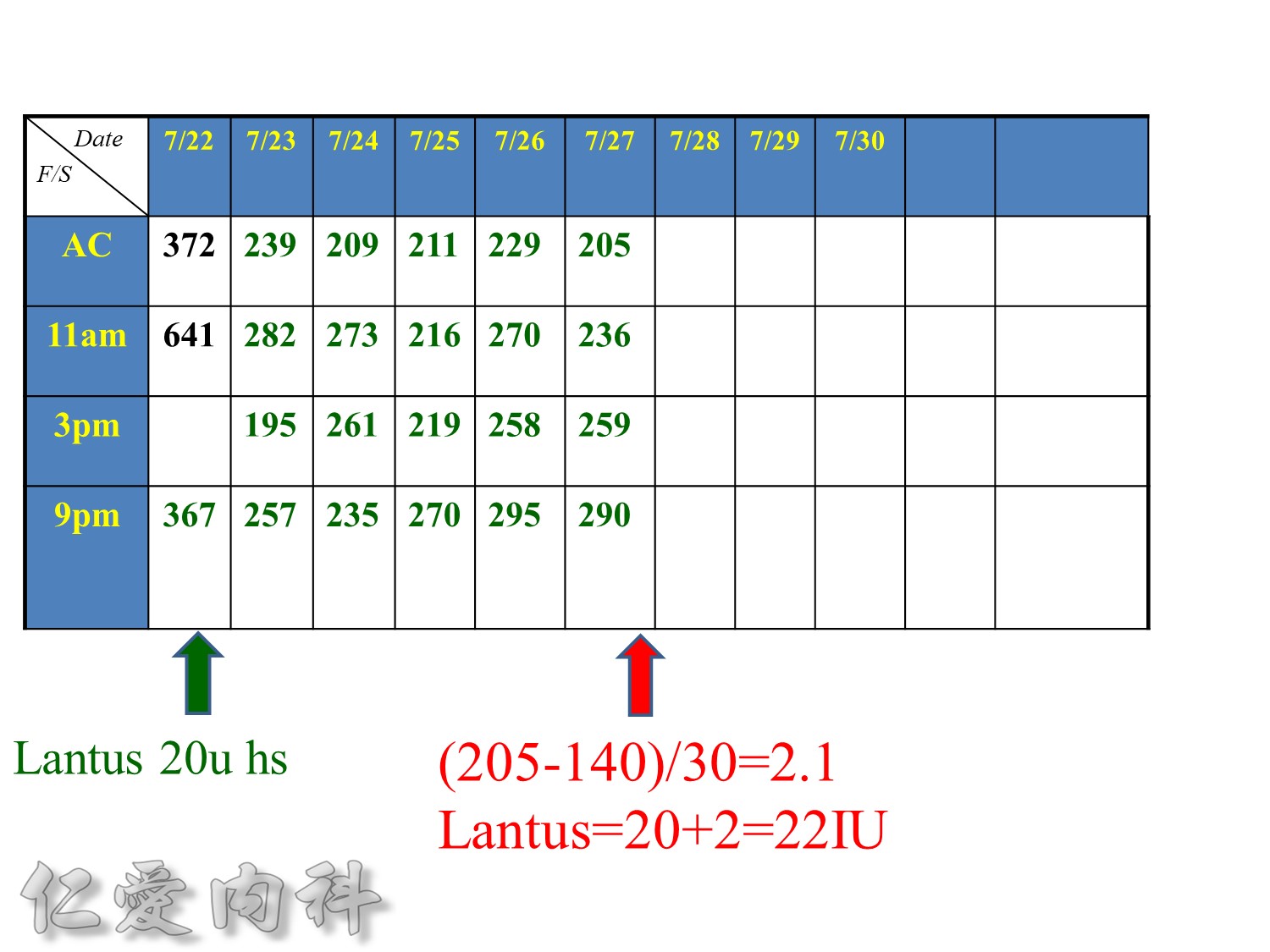
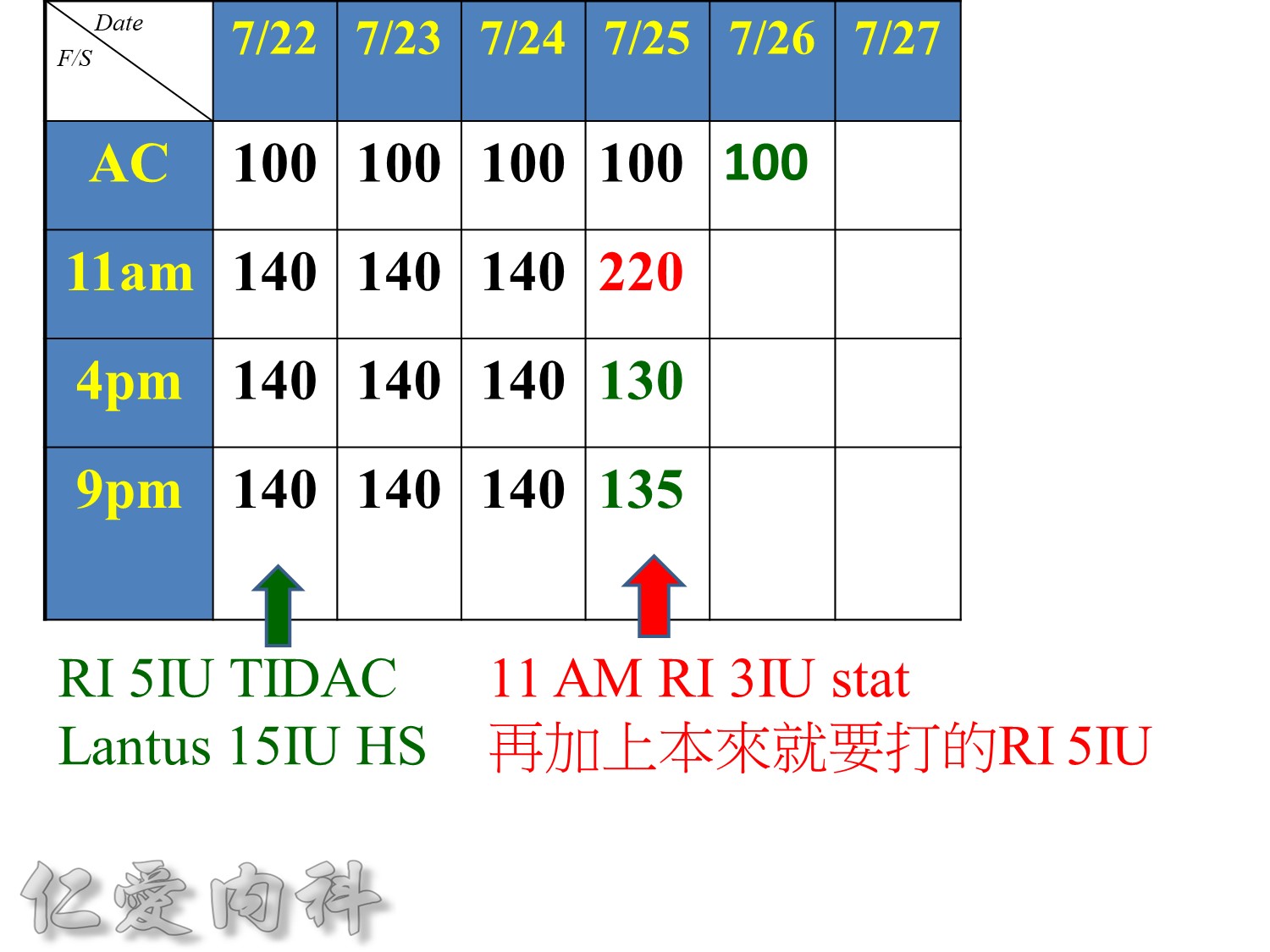
增加2U basal insulin ==> better blood sugar control


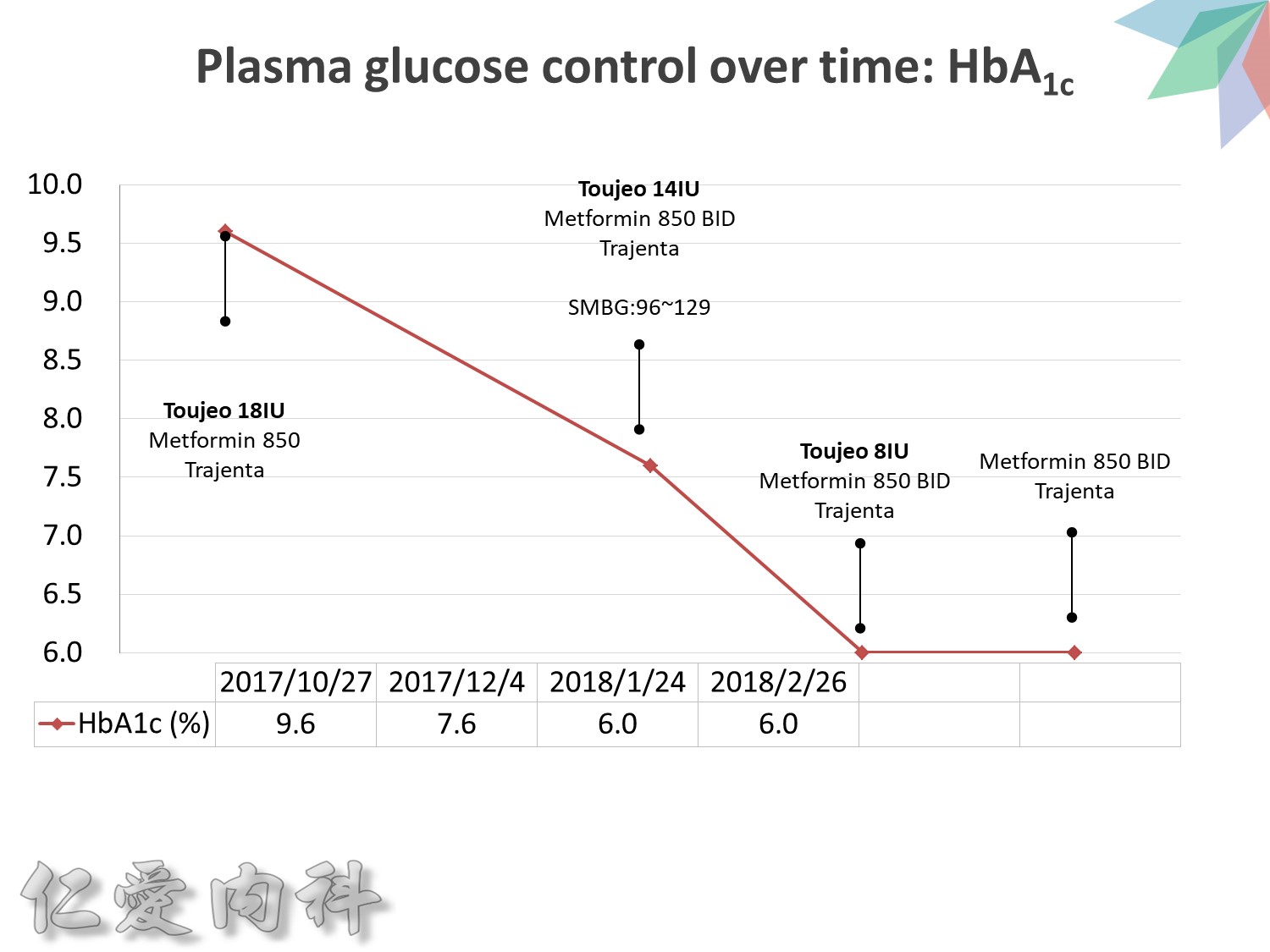

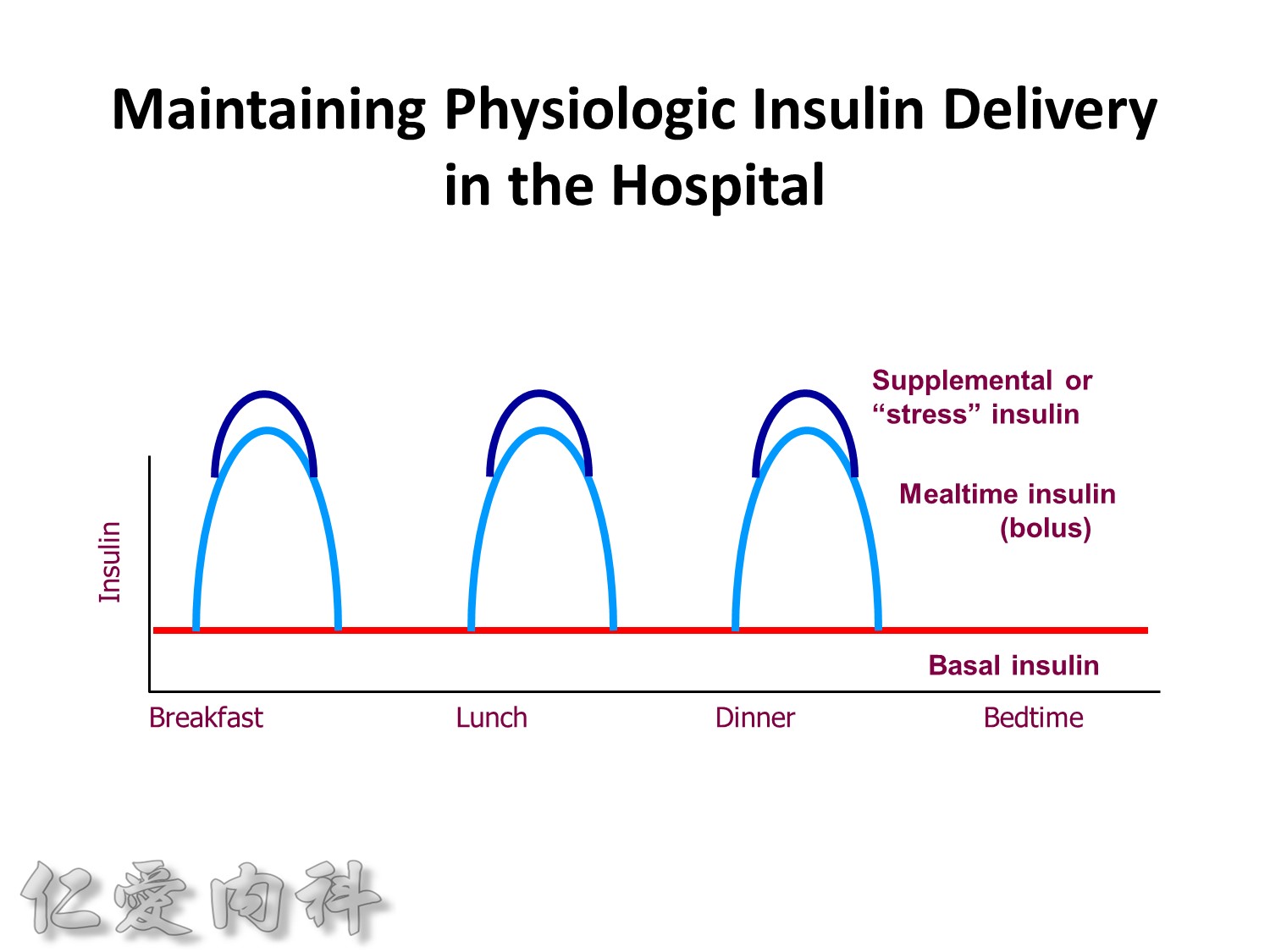
Maintaining Physiologic Insulin Delivery in the Hospital
Basal insulin
- Match insulin administration with hepatic glucose production
- Usually constant levels (20%–30% of patients have Dawn Phenomenon)
Bolus insulin (mealtime or prandial)
- Match insulin to planned carbohydrate intake
Supplemental insulin
- Correct high blood sugars to premeal target
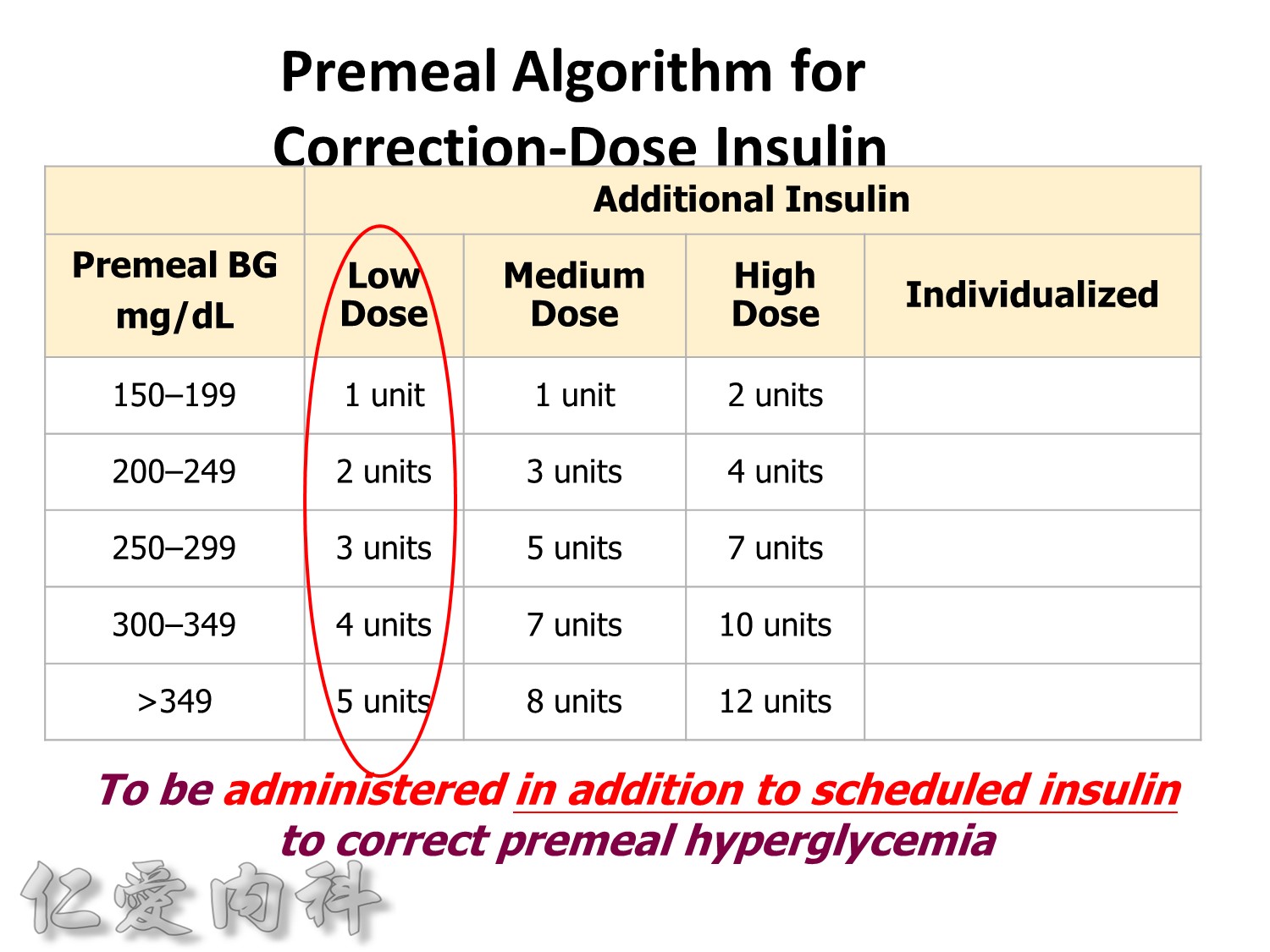
Premeal Algorithm for Correction Dose Insulin
- Correction dose algorithms for hyperglycemia obviously would be important. This is an example of a preprinted correction dose algorithm that can be provided, and it is easily implemented because all you have to do is to check which column we would like to utilize. Is it a low dose, medium dose, or high dose? If you would like to calculate the correction dose, there is that opportunity.




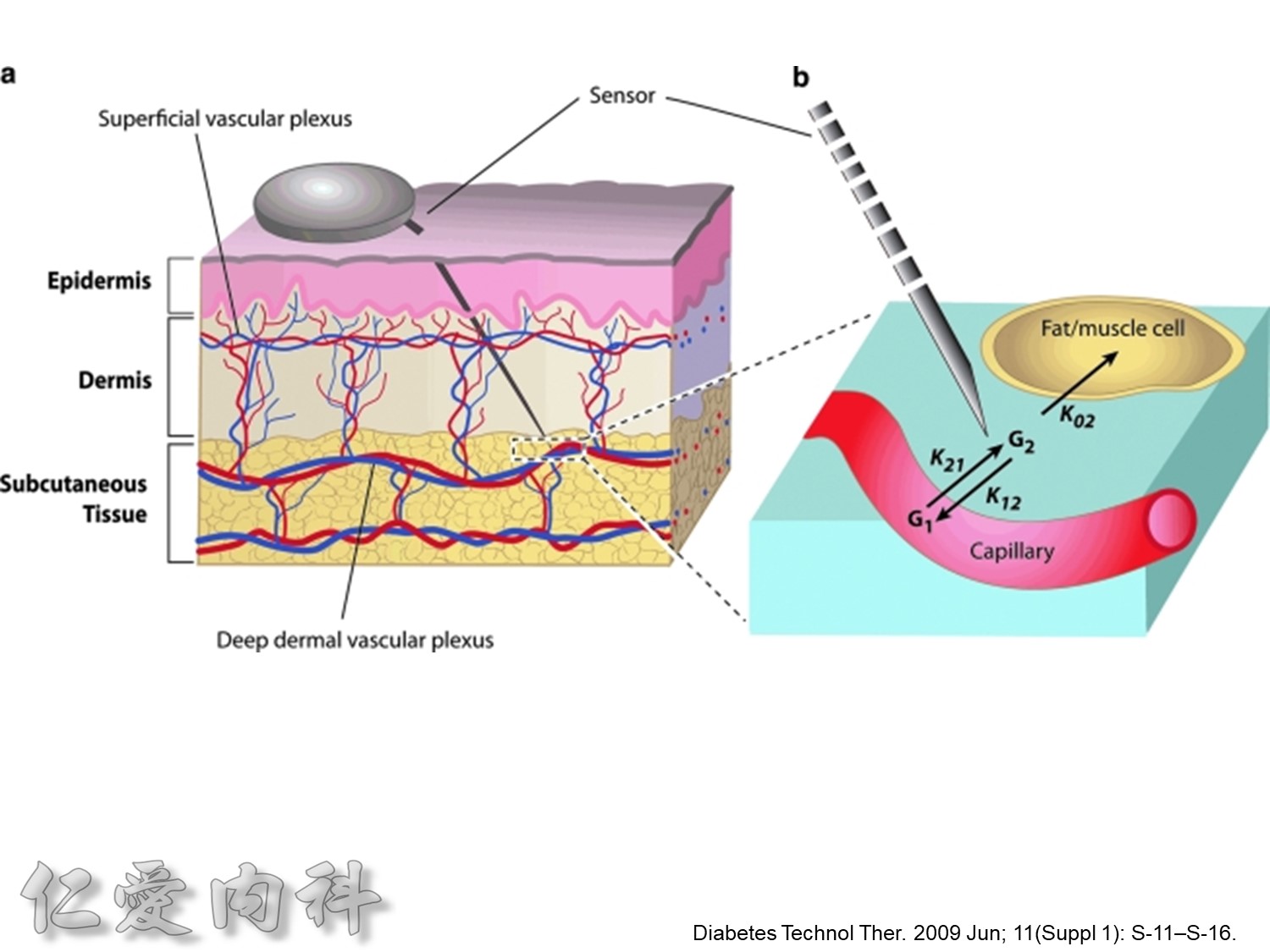
http://www.diabetesforecast.org/2013/jun/is-plasma-glucose-a-better-measure.html
The reported gradient between interstitial and plasma glucose concentrations has varied between 20%to 110%.


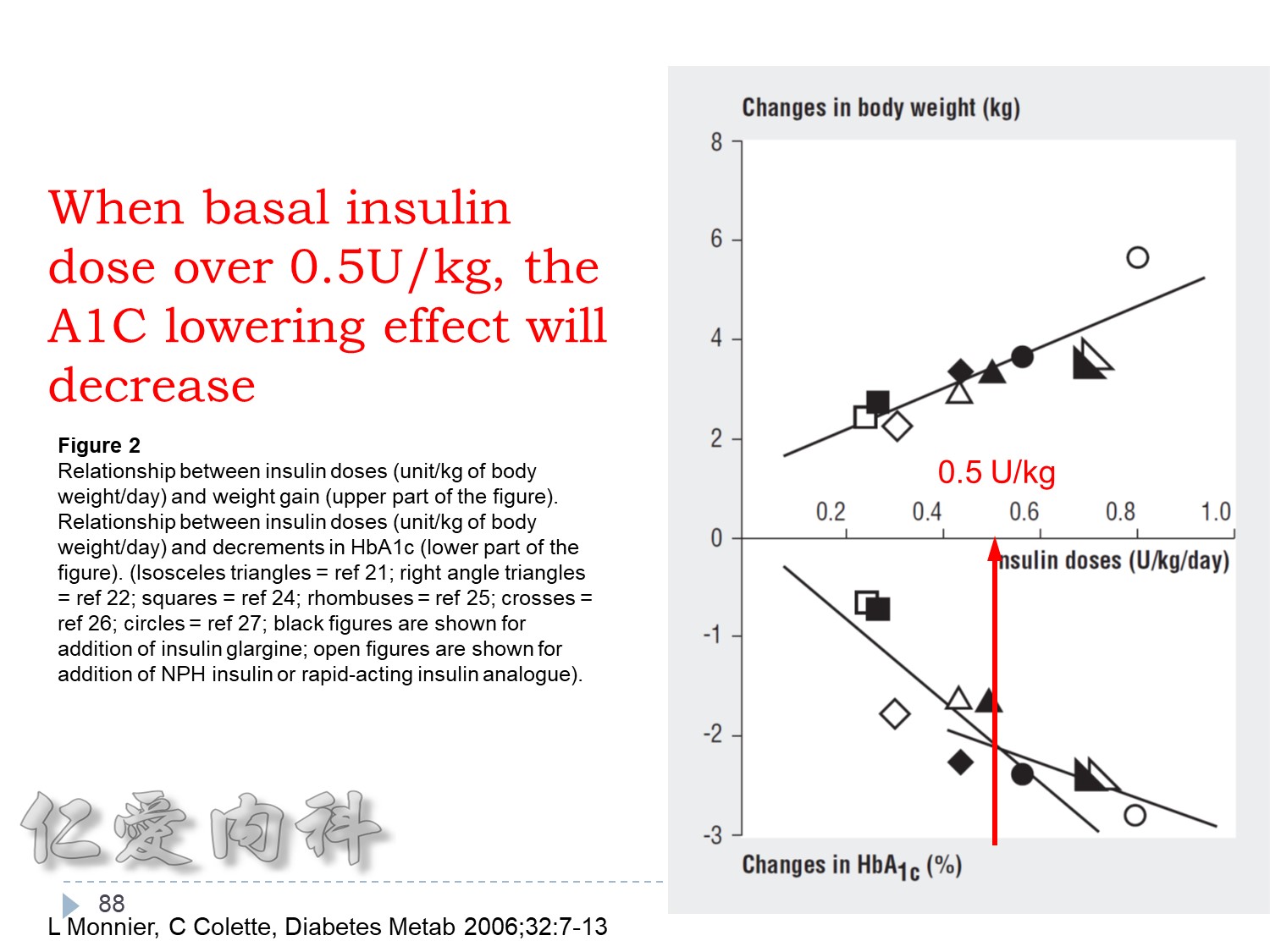
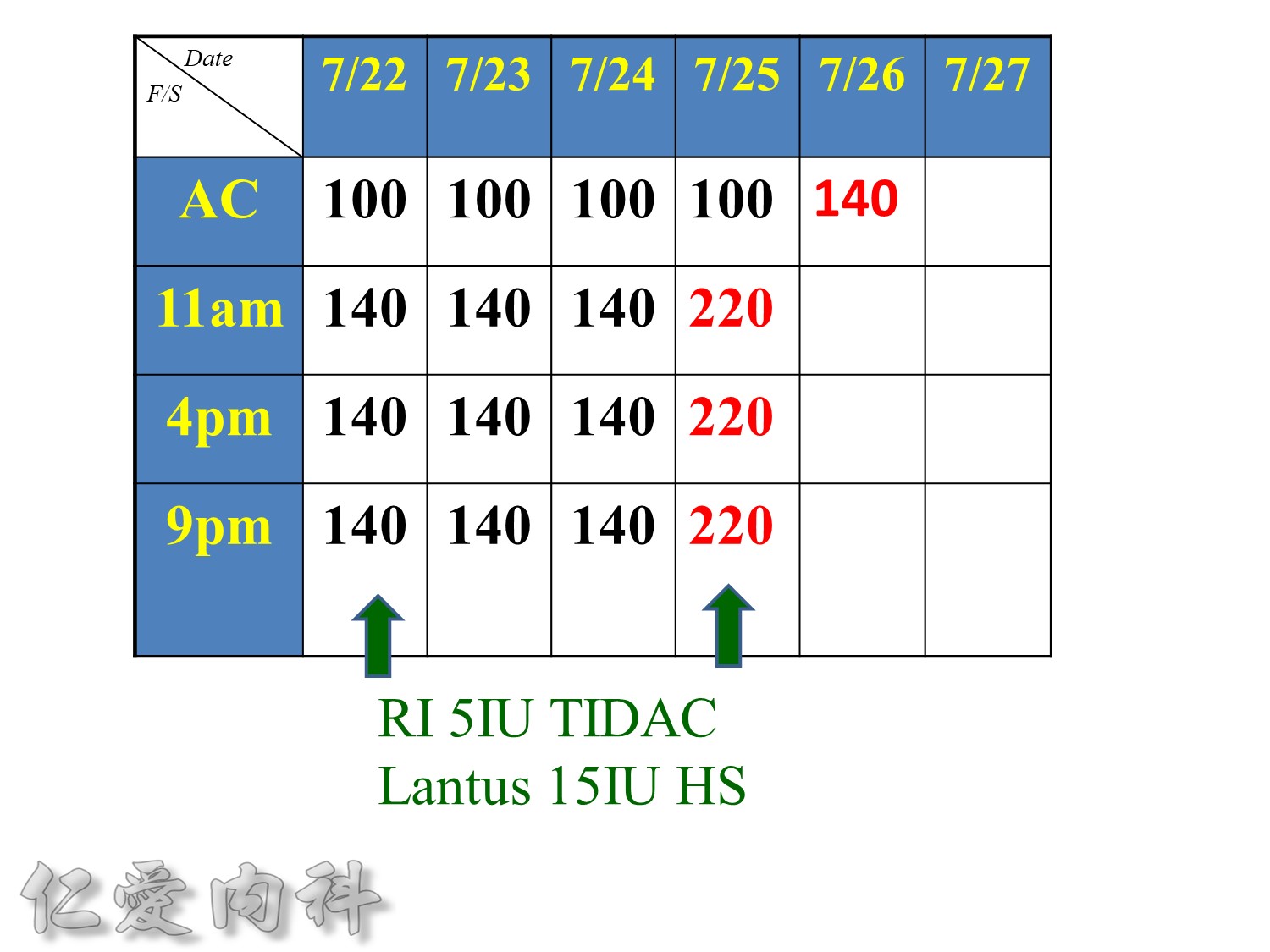
>當Basal insulin 使用>0.6 IU/kg==> 效果不好

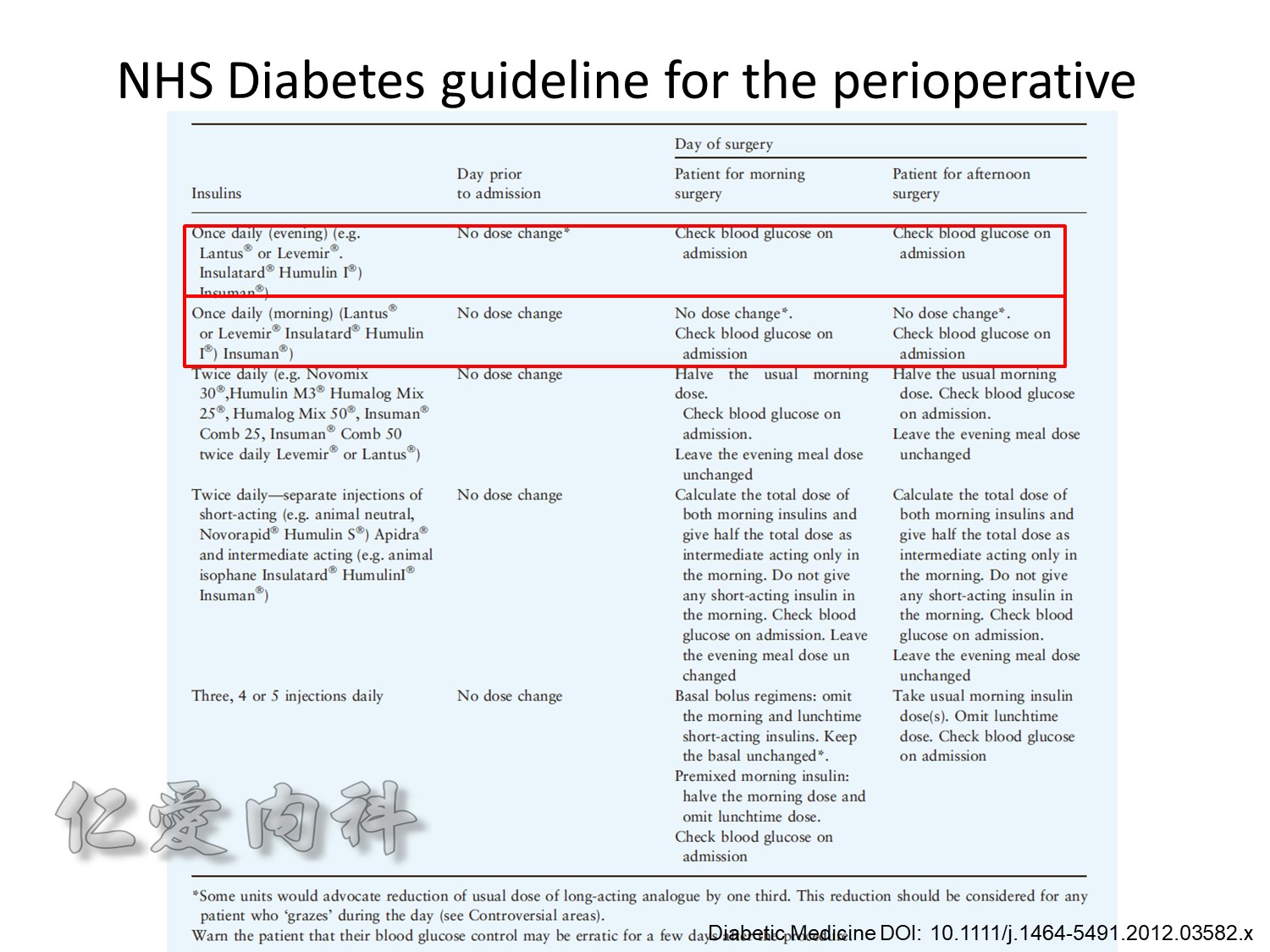
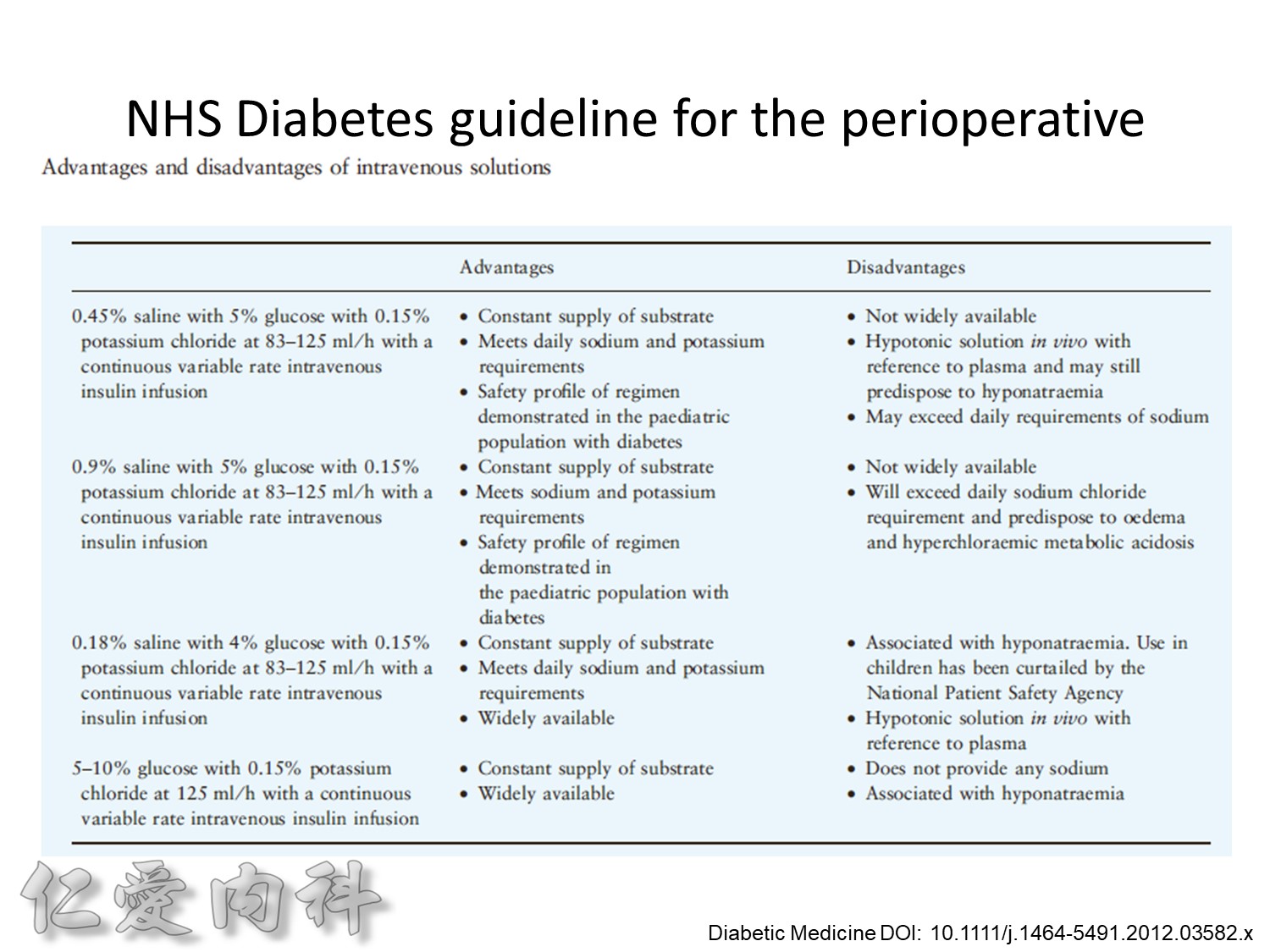

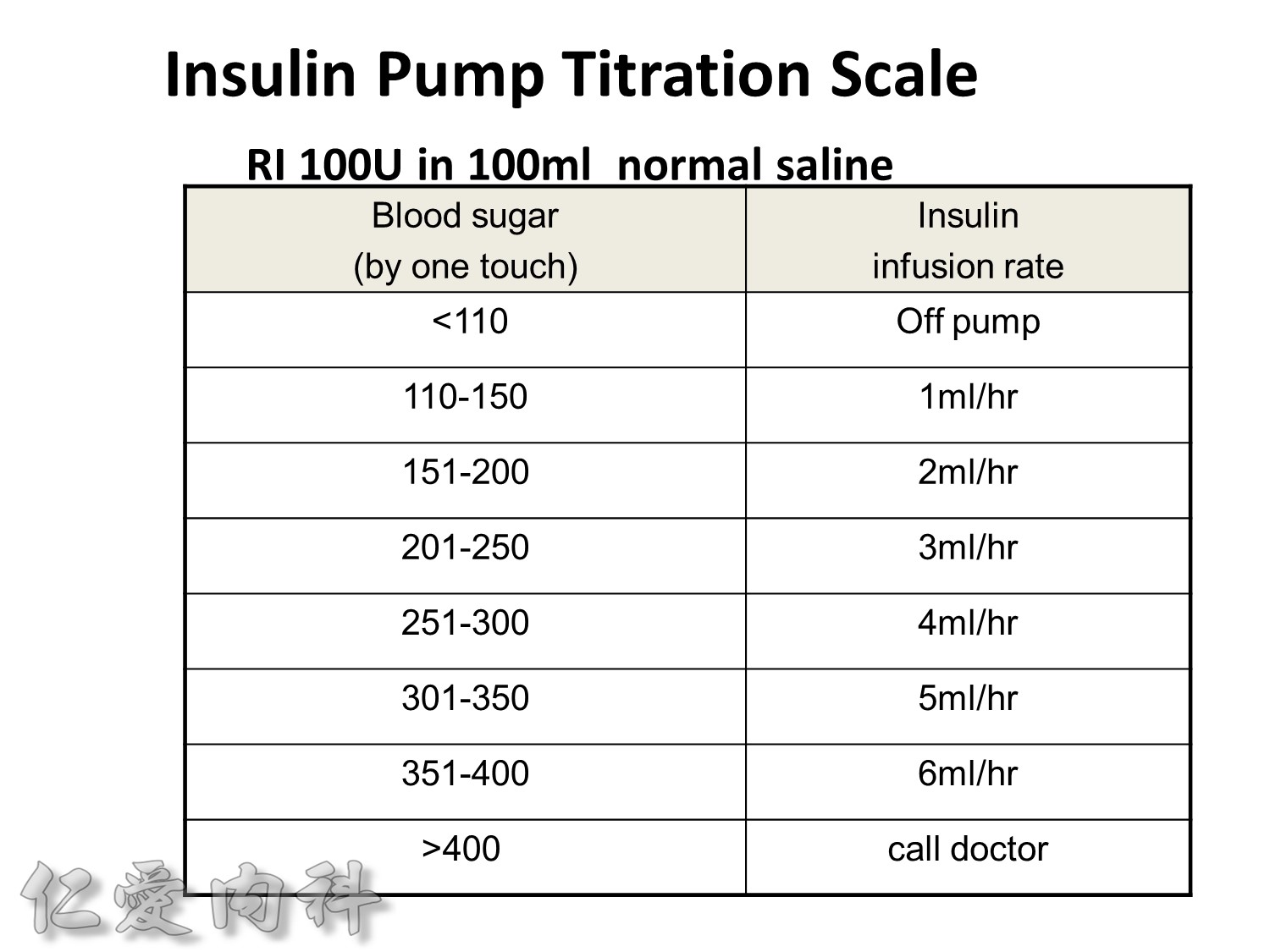
Insulin infusions can effectively control plasma glucose in the perioperative period and when the patient is unable to take anything by mouth. The absorption of subcutaneous insulin may be variable in such situations. Regular insulin is preferred over insulin analogues for IV insulin infusion since it is less expensive and equally effective. The physician must consider carefully the clinical setting in which an insulin infusion will be utilized, including whether adequate ancillary personnel are available to monitor the plasma glucose frequently and whether they can adjust the insulin infusion rate to maintain the plasma glucose within the optimal range.
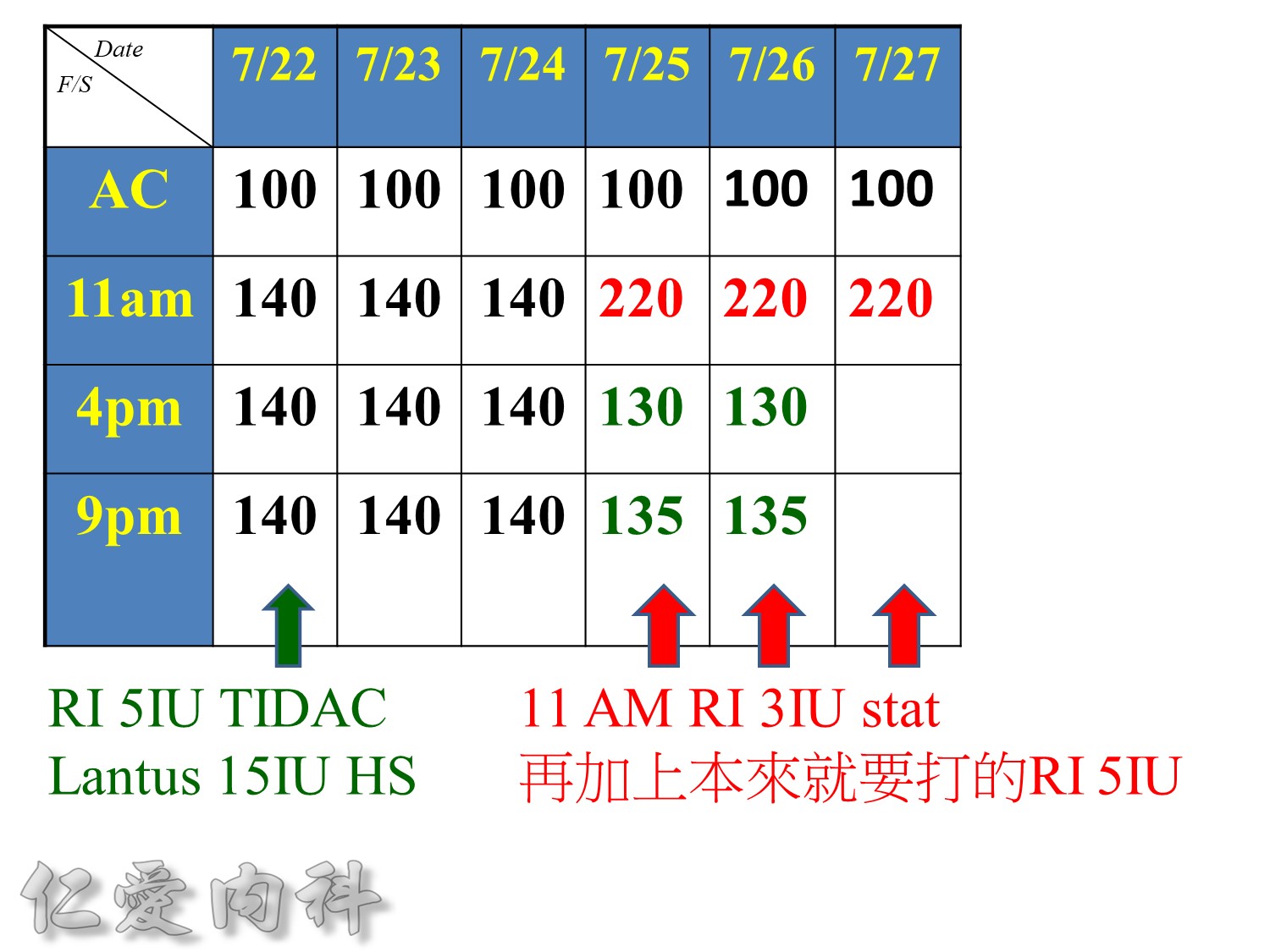
Because of the short half-life of IV regular insulin, it is necessary to administer long-acting insulin prior to discontinuation of the insulin infusion to avoid a period of insulin deficiency. An alternative to an insulin infusion is basal or “scheduled” insulin provided by SC, long-acting insulin supplemented by prandial or “corrective” insulin using a short-acting insulin (insulin analogues preferred). The use of “sliding scale” insulin alone, where no insulin is given unless the blood glucose is elevated, is inadequate for in-patient glucose management and should not be used. The short-acting, pre-prandial insulin dose should include coverage for food consumption (based on anticipated carbohydrate intake) plus a corrective or supplemental insulin based on the patient’s insulin sensitivity and the blood glucose. For example, if the patient is thin (and likely insulin sensitive), a corrective insulin supplement might be 1 unit for each 2.7 mmol/L (50 mg/dL) over the glucose target. If the patient is obese and insulin resistant, then the insulin supplement might be 2 units for each 2.7 mmol/L (50 mg/dL) over the glucose target. It is critical to individualize the regimen and adjust the basal or “scheduled” insulin dose frequently, based on the corrective insulin required.
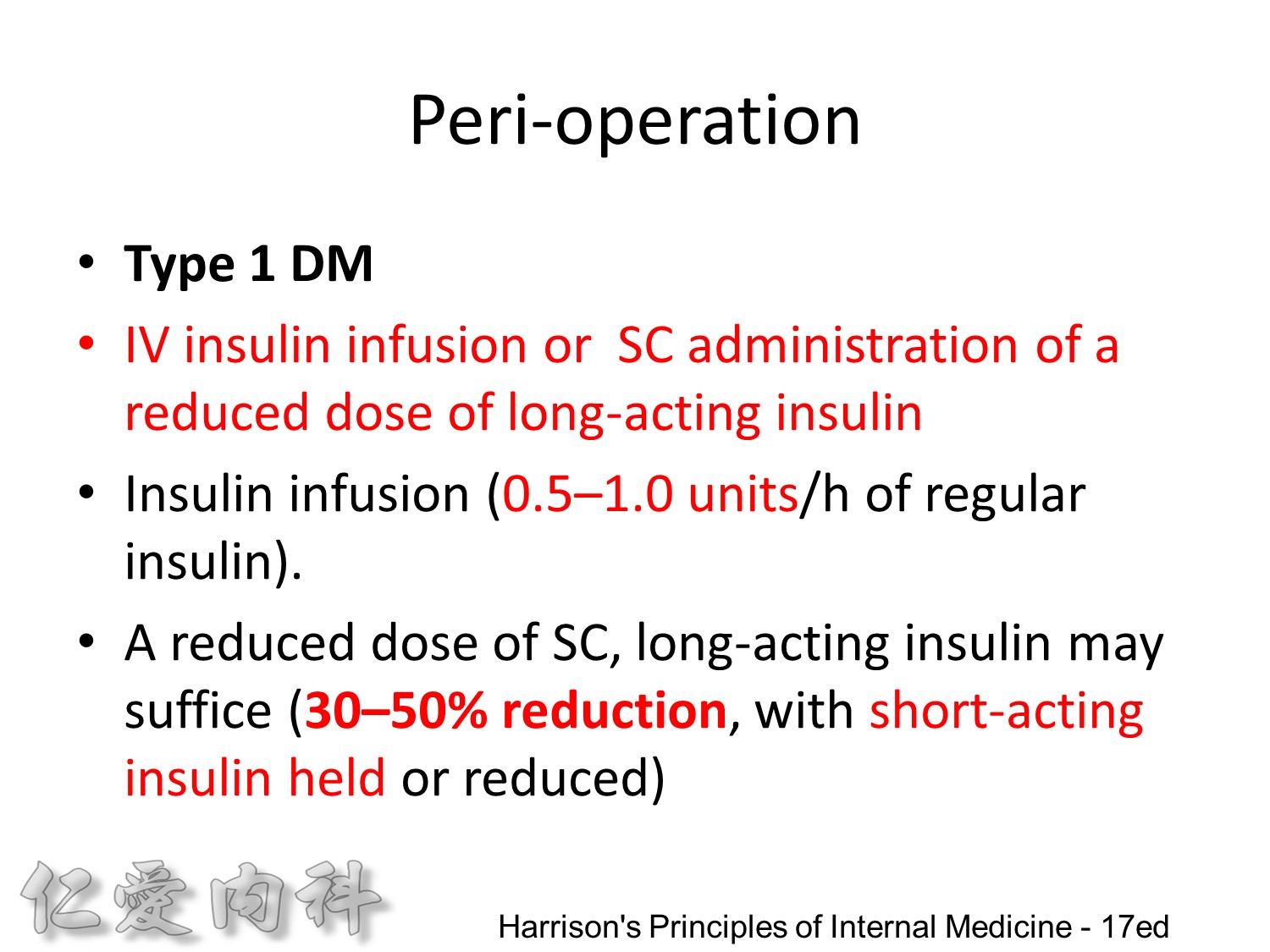
Individuals with type 1 DM who are undergoing general anesthesia and surgery, or who are seriously ill, should receive continuous insulin, either through an IV insulin infusion or by SC administration of a reduced dose of long-acting insulin. Short-acting insulin alone is insufficient. Prolongation of a surgical procedure or delay in the recovery room is not uncommon and may result in periods of insulin deficiency leading to DKA. Insulin infusion is the preferred method for managing patients with type 1 DM in the perioperative period or when serious concurrent illness is present (0.5–1.0 units/h of regular insulin). Insulin-infusion algorithms jointly developed and implemented by nursing and physician staff are advised. If the diagnostic or surgical procedure is brief and performed under local or regional anesthesia, a reduced dose of SC, long-acting insulin may suffice (30–50% reduction, with short-acting insulin held or reduced). This approach facilitates the transition back to long-acting insulin after the procedure. Glucose may be infused to prevent hypoglycemia. The blood glucose should be monitored frequently during the illness or in the perioperative period.
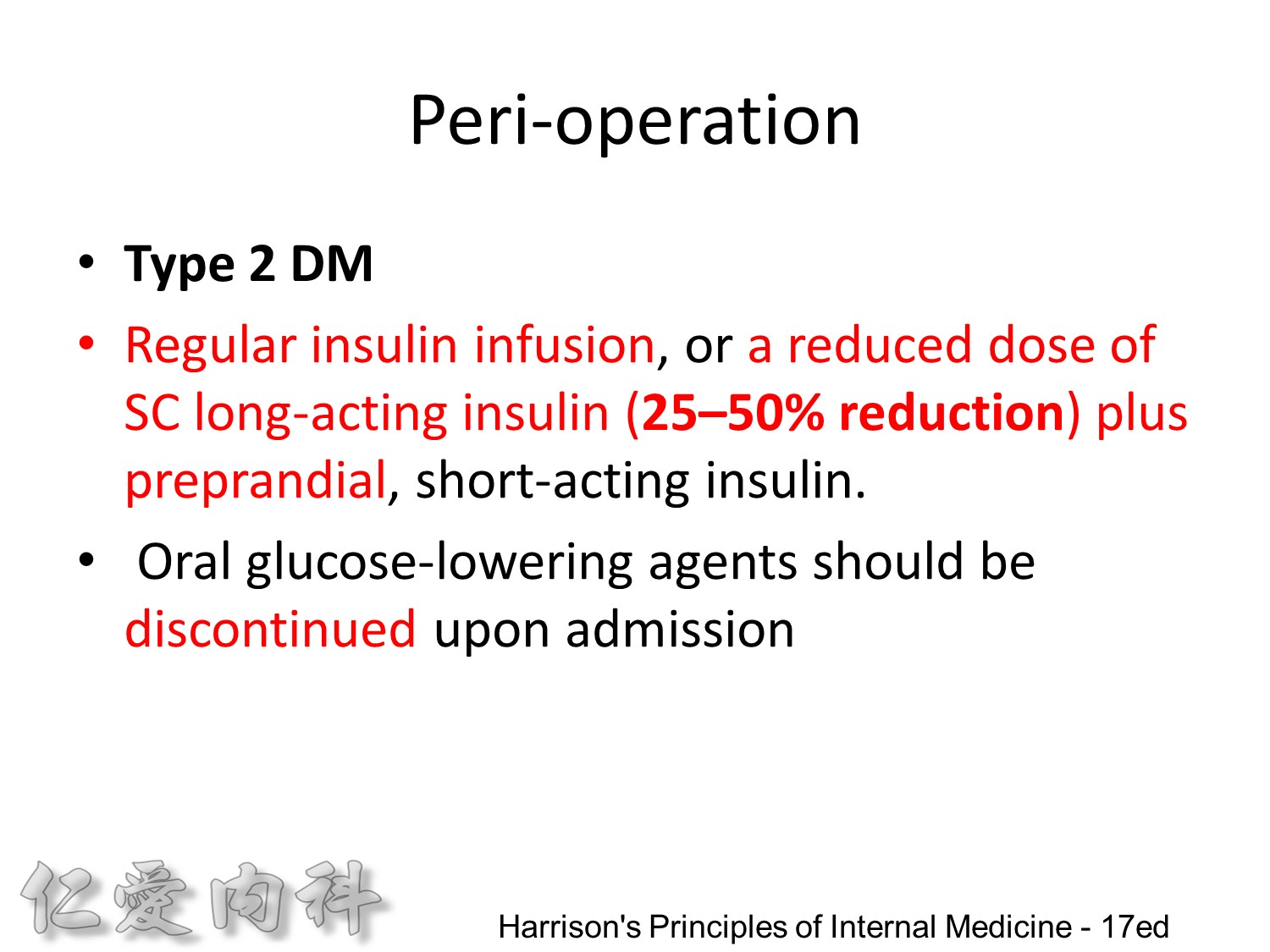
Individuals with type 2 DM can be managed with either regular insulin infusion, or a reduced dose of SC long-acting insulin (25–50% reduction) plus preprandial, short-acting insulin. Oral glucose-lowering agents should be discontinued upon admission and are not useful in regulating the plasma glucose in clinical situations where the insulin requirements and glucose intake are changing rapidly. Moreover, these oral agents may be dangerous if the patient is fasting (e.g., hypoglycemia with sulfonylureas). Metformin should be withheld when radiographic contrast media will be given or if severe CHF, acidosis, or declining renal function is present.

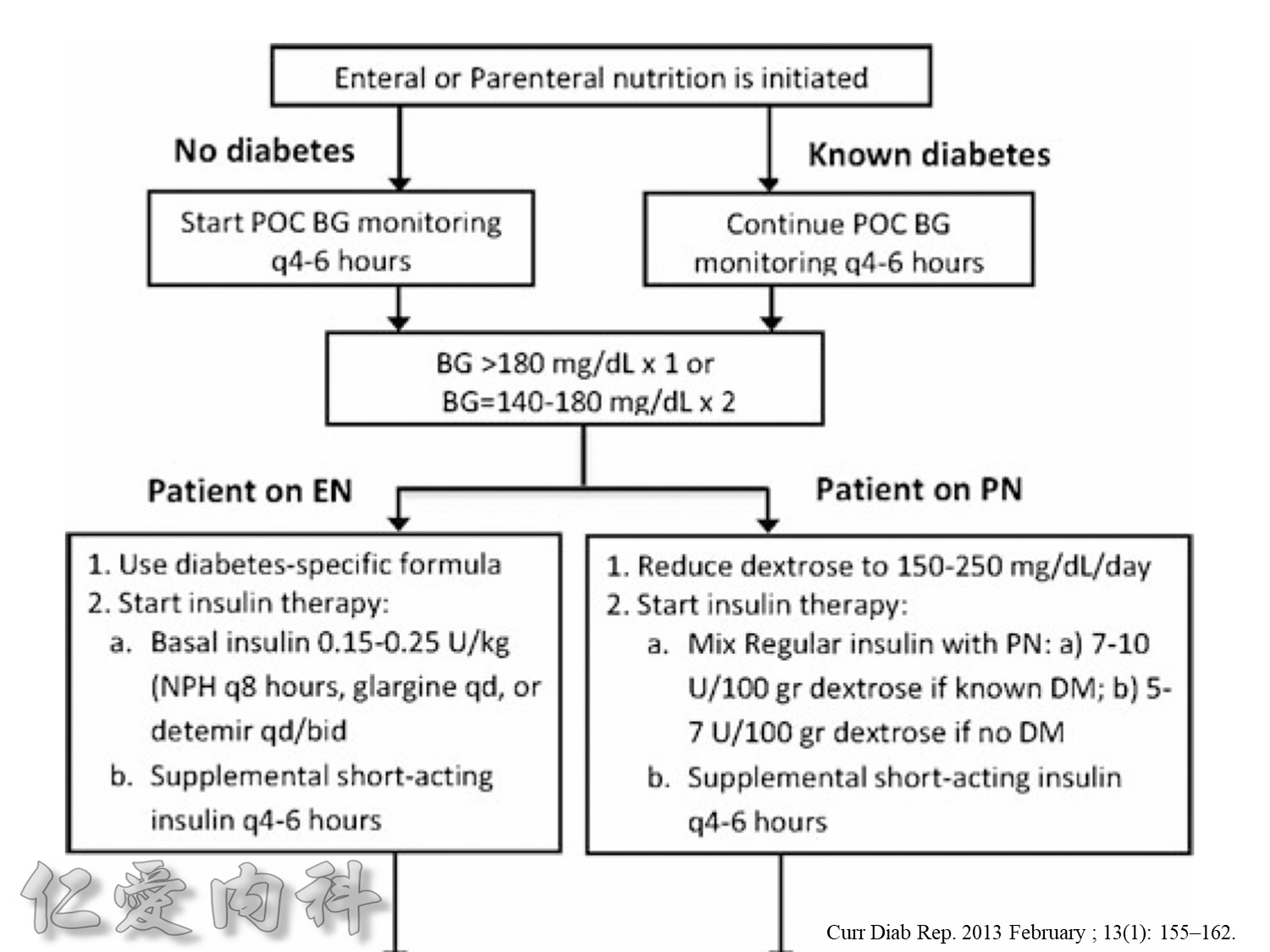
Taita No 5 約略是D10W –>10g dextrose in 100ml water, for Taita No.5 400ml
==>約有40 g dextrose
所以若+RI 4U 至 Taita No 5 是合理的
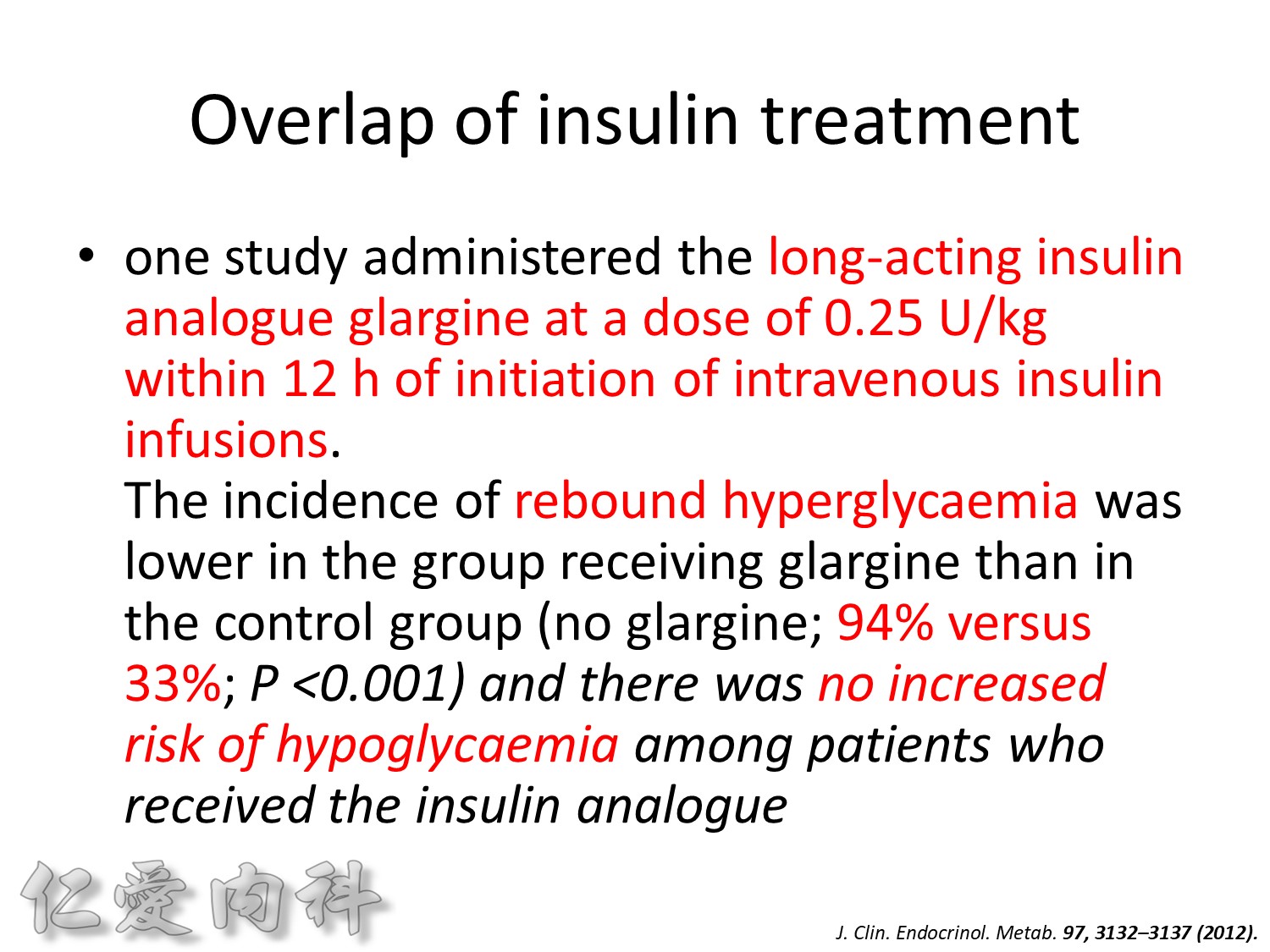
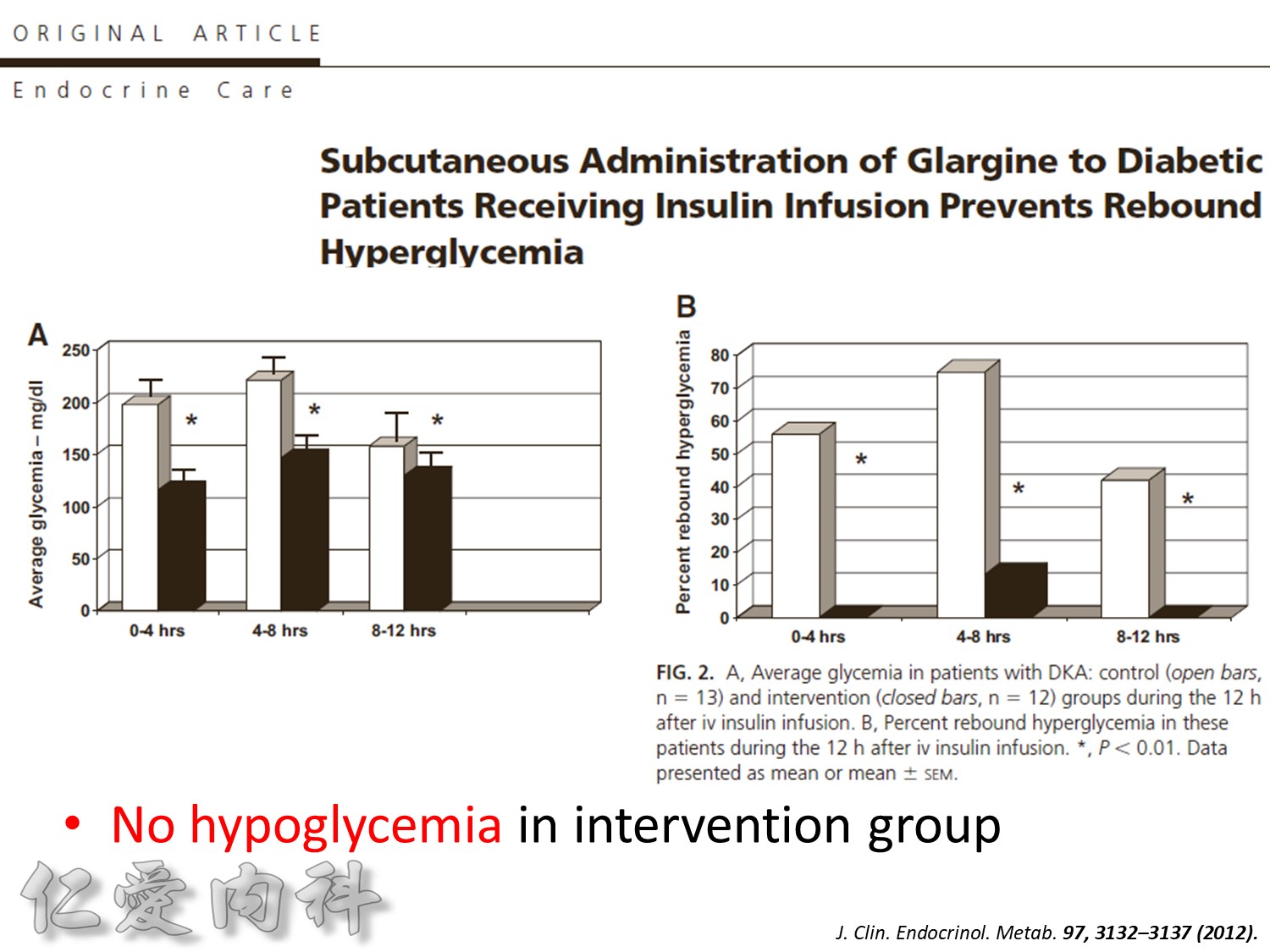
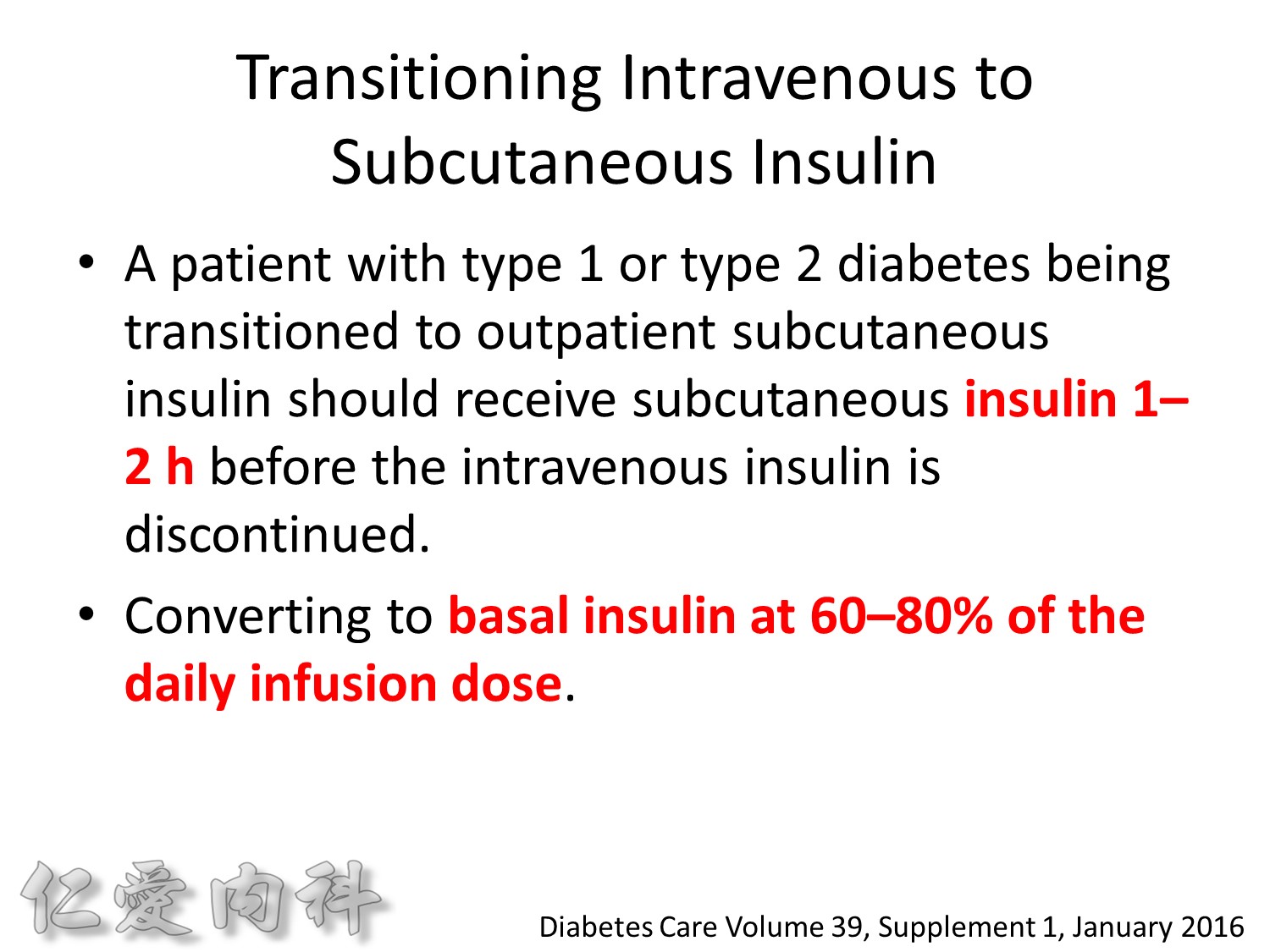


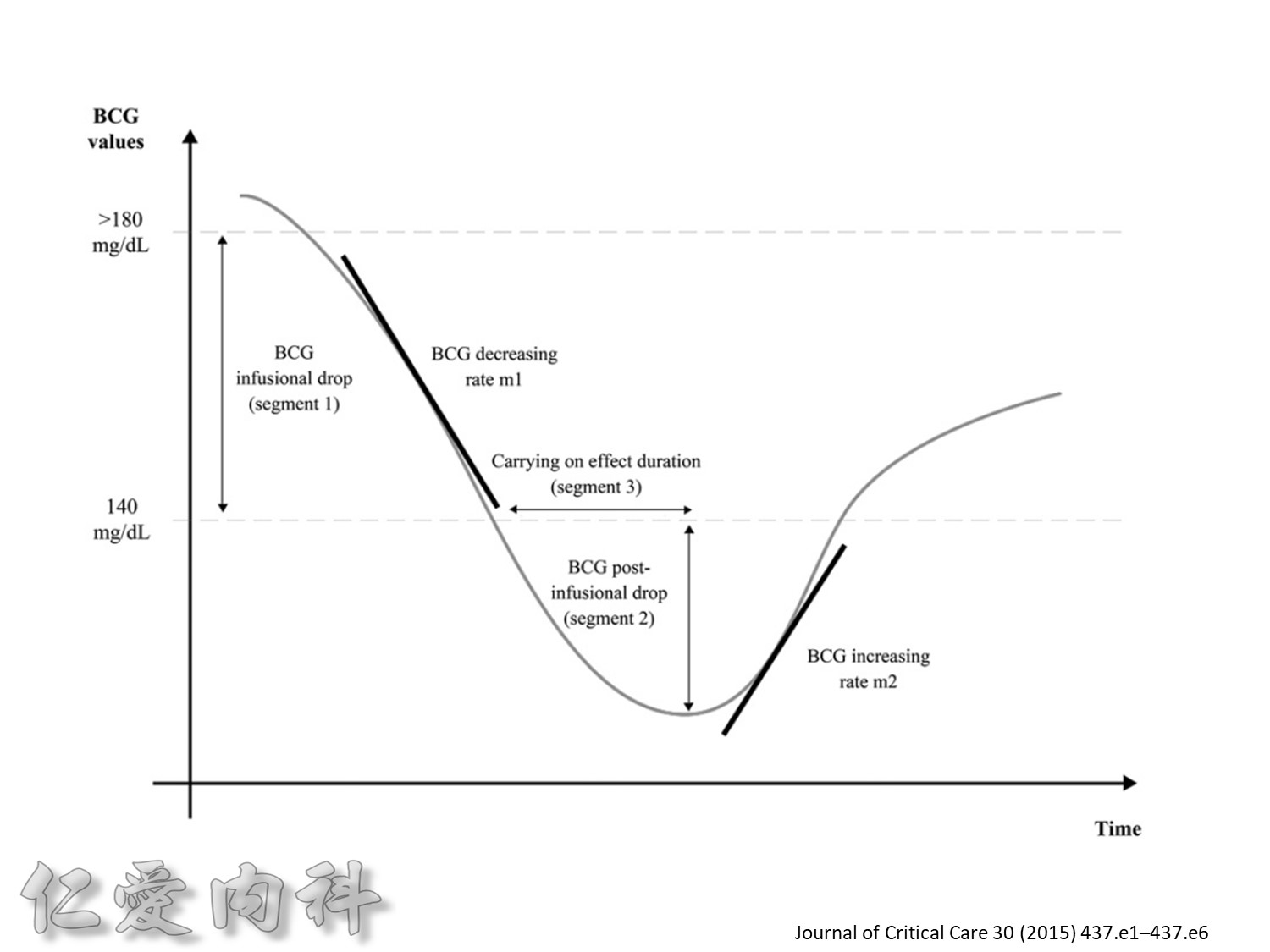
Fig. 1. The primary outcomemeasure was the extent of carryover effect (segment 2/segment 1). Secondary end pointmeasures were rate of BGC reduction during insulin infusion (slopem1),
duration of the carryover effect (segment 3), and the rate of BGC increase from lowest BGC value to the first BGC value greater than or equal to 140 mg/dL (slope m2).
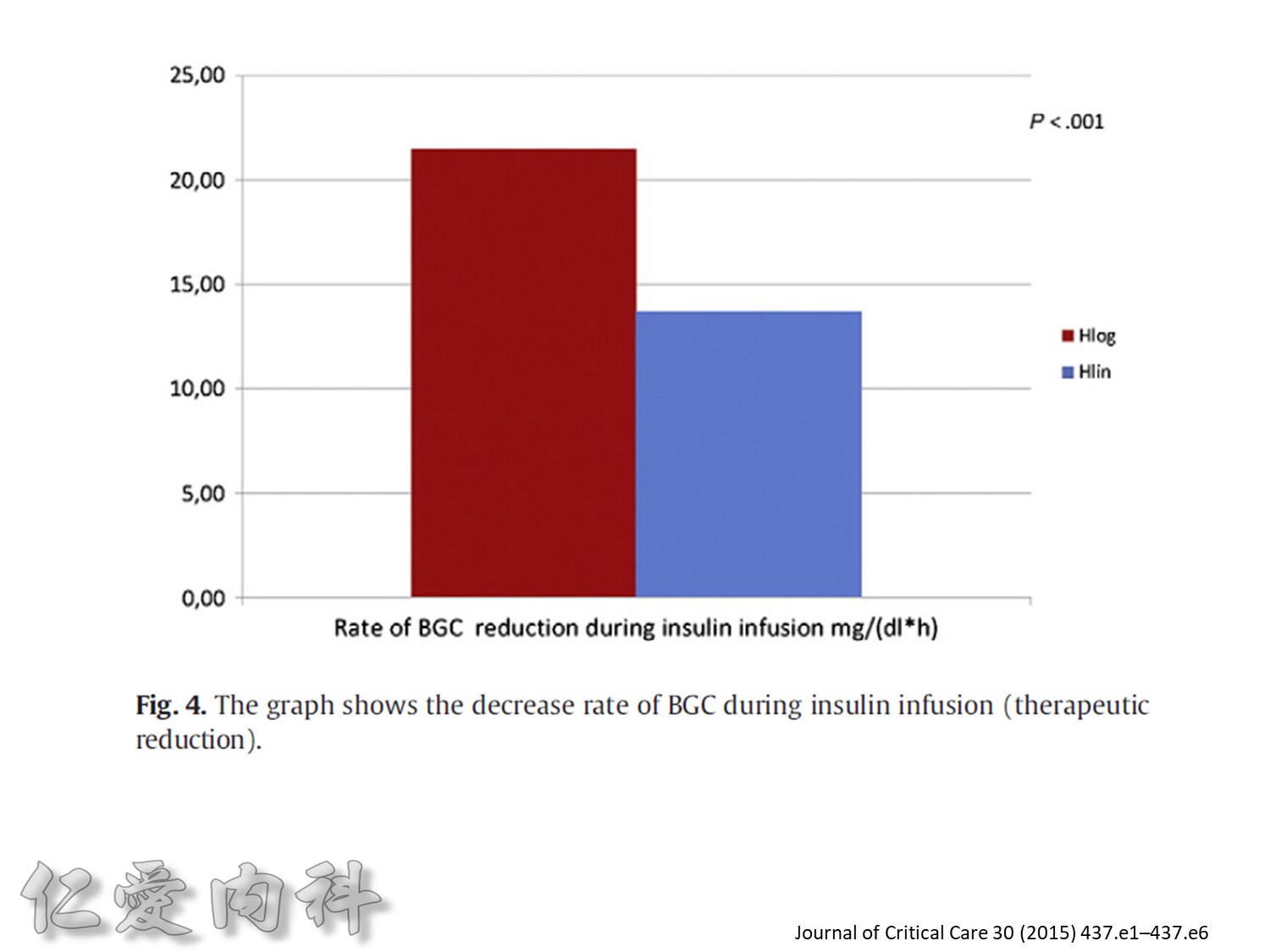
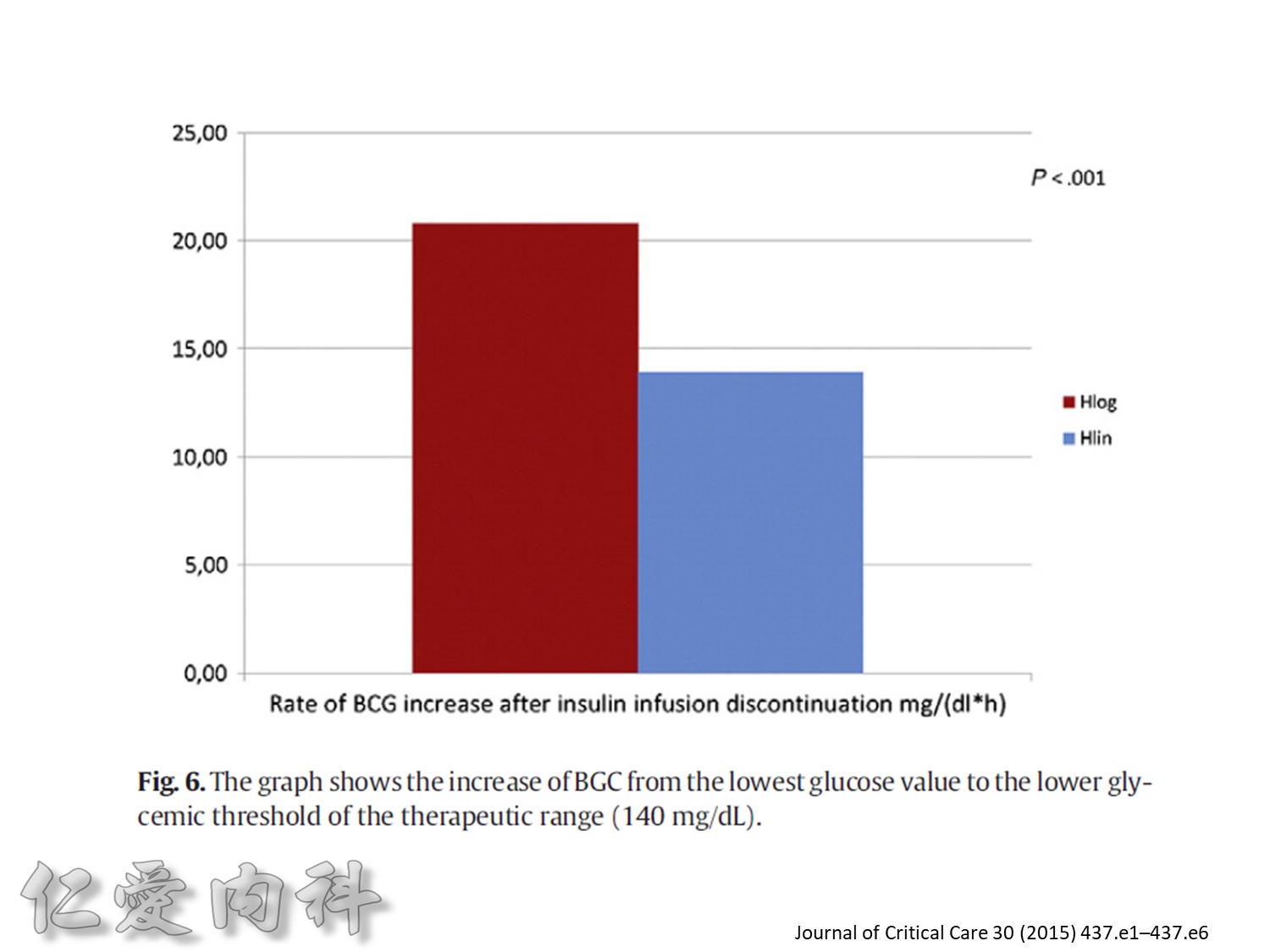
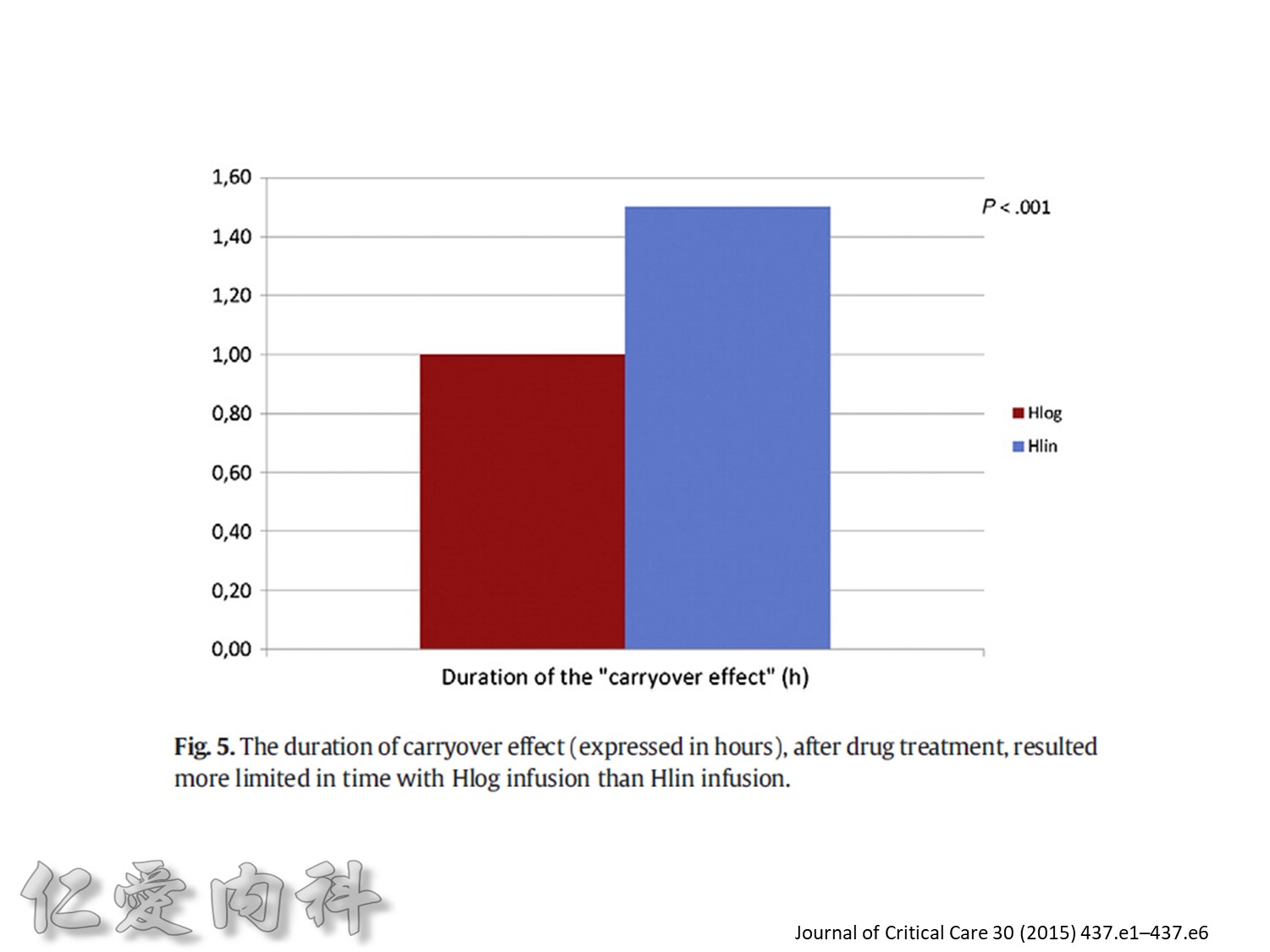
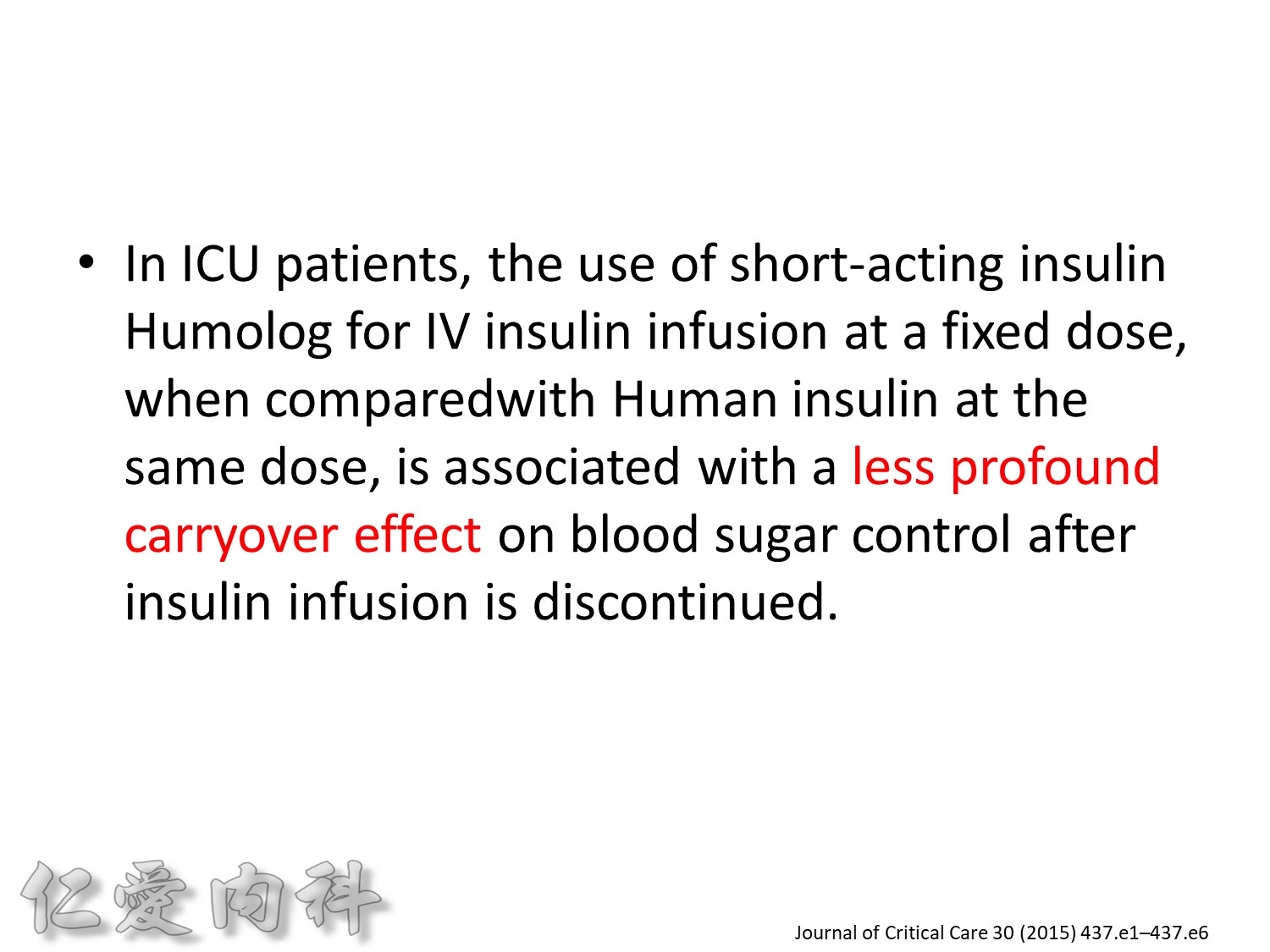
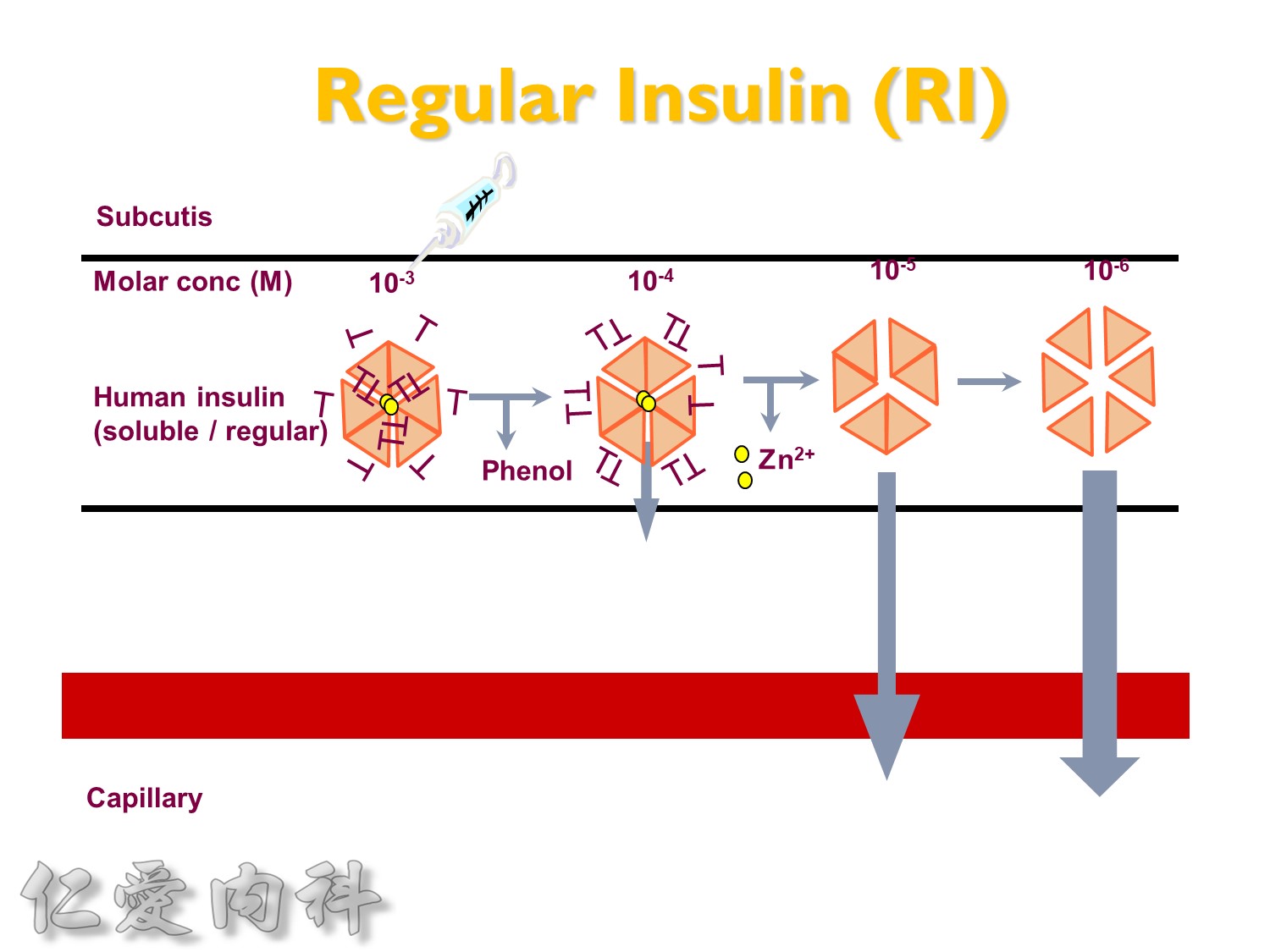
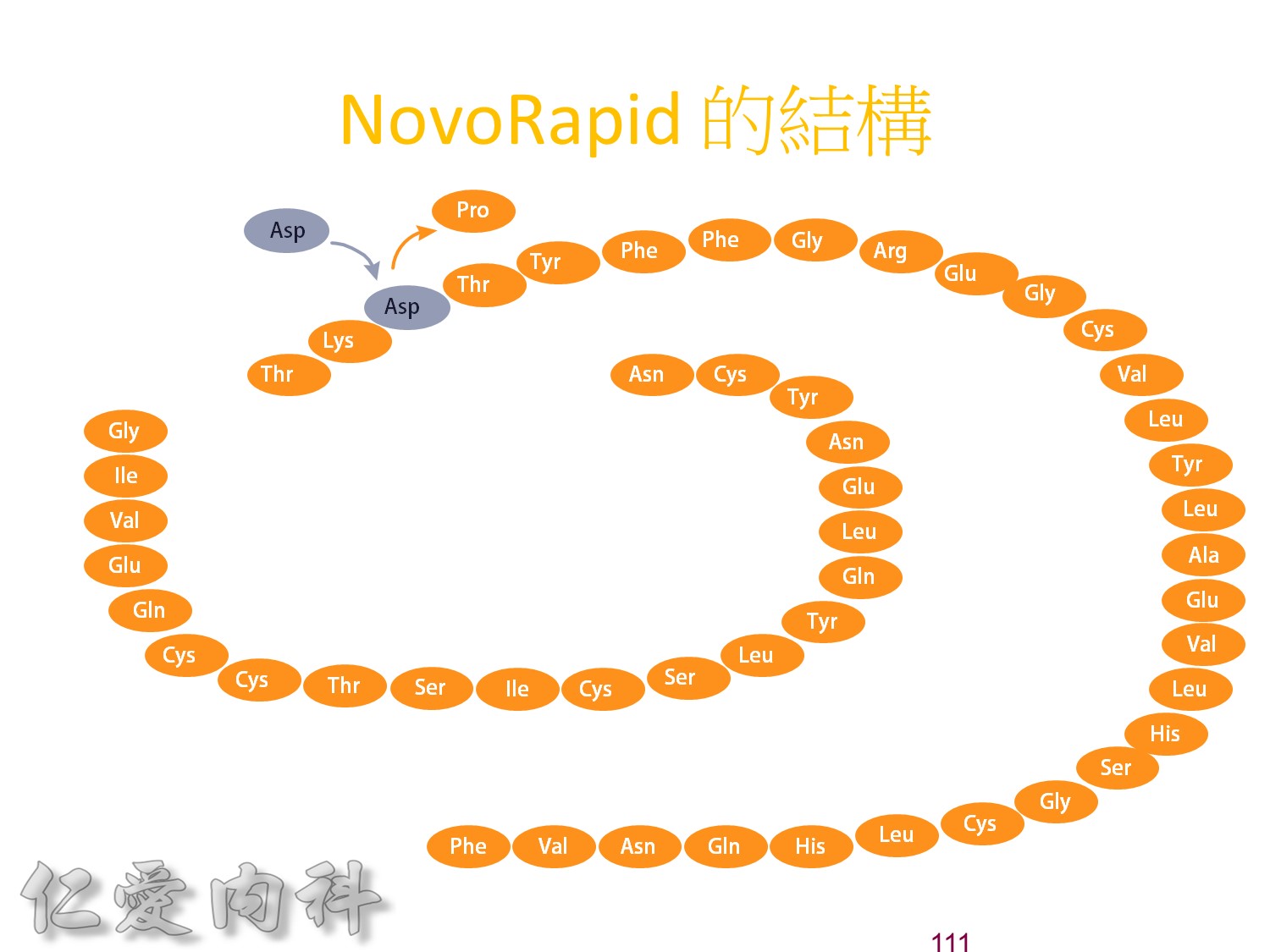
為了改善human insulin這樣的特性, 將人類胰島素分子結構第B28位置上的Proline以基因工程的方式置換為Aspartic Acid。
