講者: 楊文萍 醫師
校閱: Ian YC Chen, MD
上次校閱: 2018.05.06


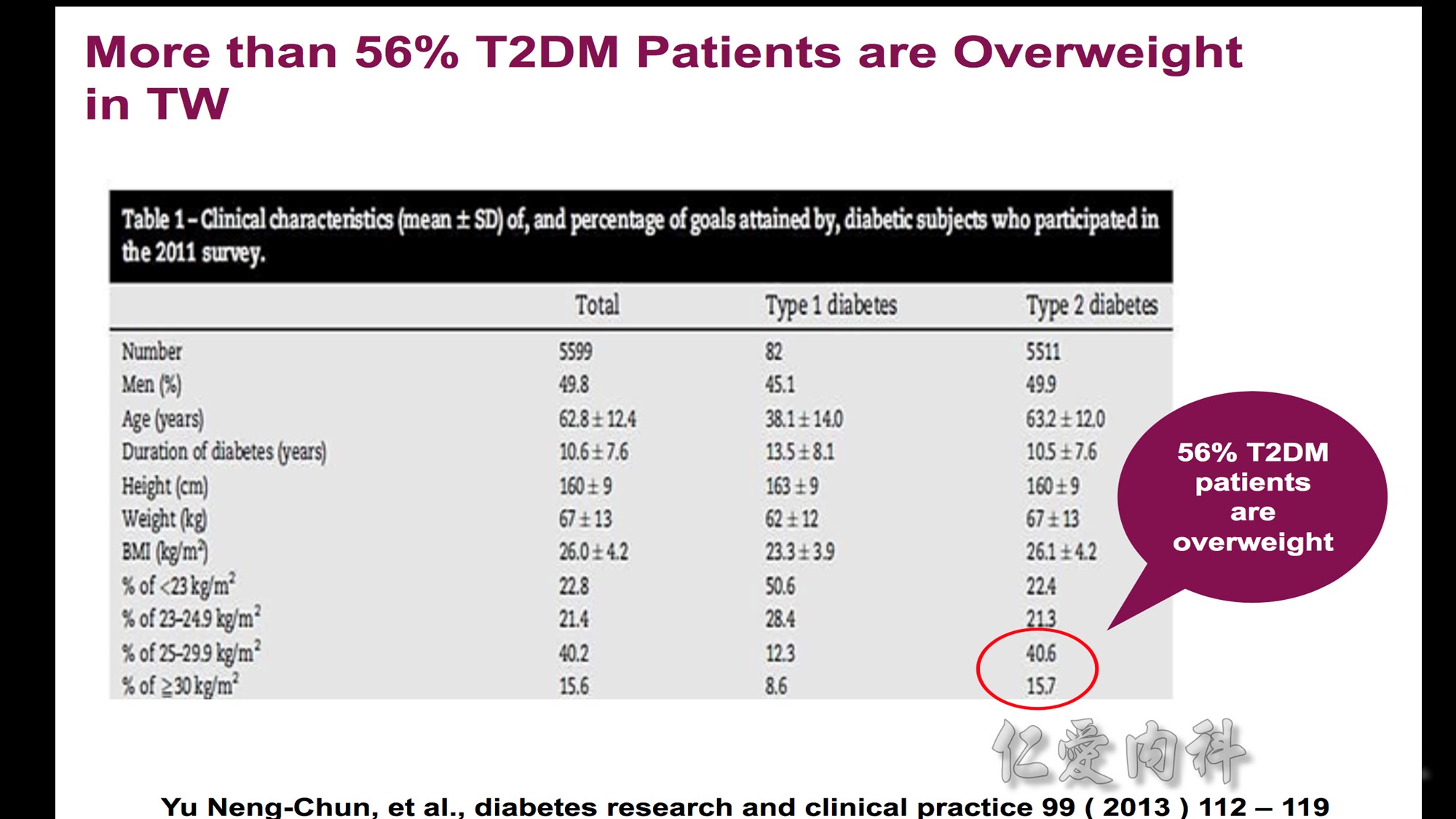




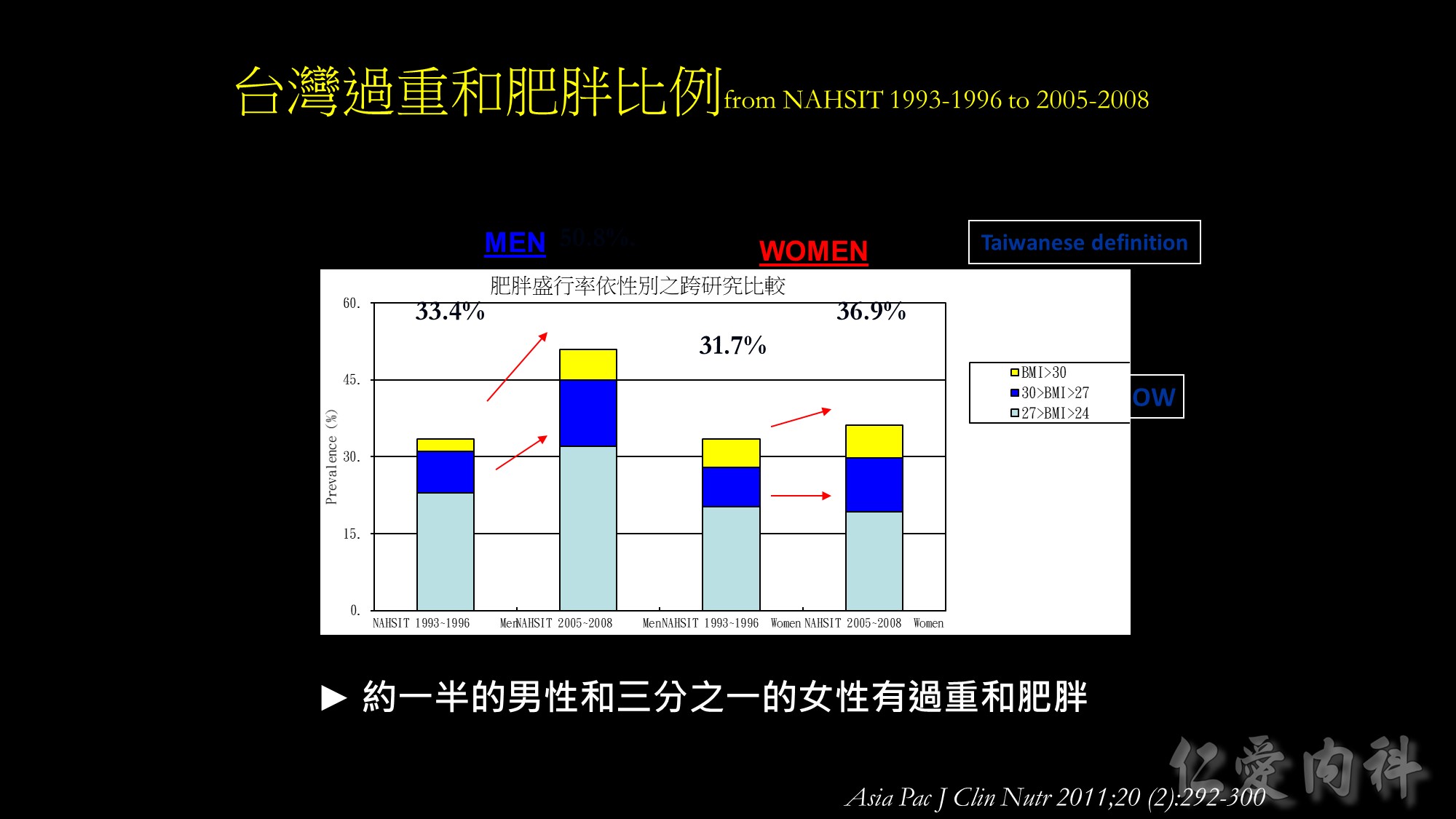
Based on the Taiwanese criteria, the prevalence of overweight and obesity increased by approximately 5% from 31.7% to 36.9% in the past 12 years among females from the Nutrition and Health Survey in Taiwan. Compared to females, males had a larger accretion in prevalence of overweight and obesity, that is from 33.4% to 50.8%. Approximately half of the men and more than one third of women are overweight and obese now in Taiwan.






In a prospective study of more than 1 million adults in the United States (457,785 men and 588,369 women), 201,622 deaths occurred during 14 years of follow-up.
The risk of death from all causes, cardiovascular disease, cancer, or other diseases increases throughout the range of moderate and severe overweight for both genders.
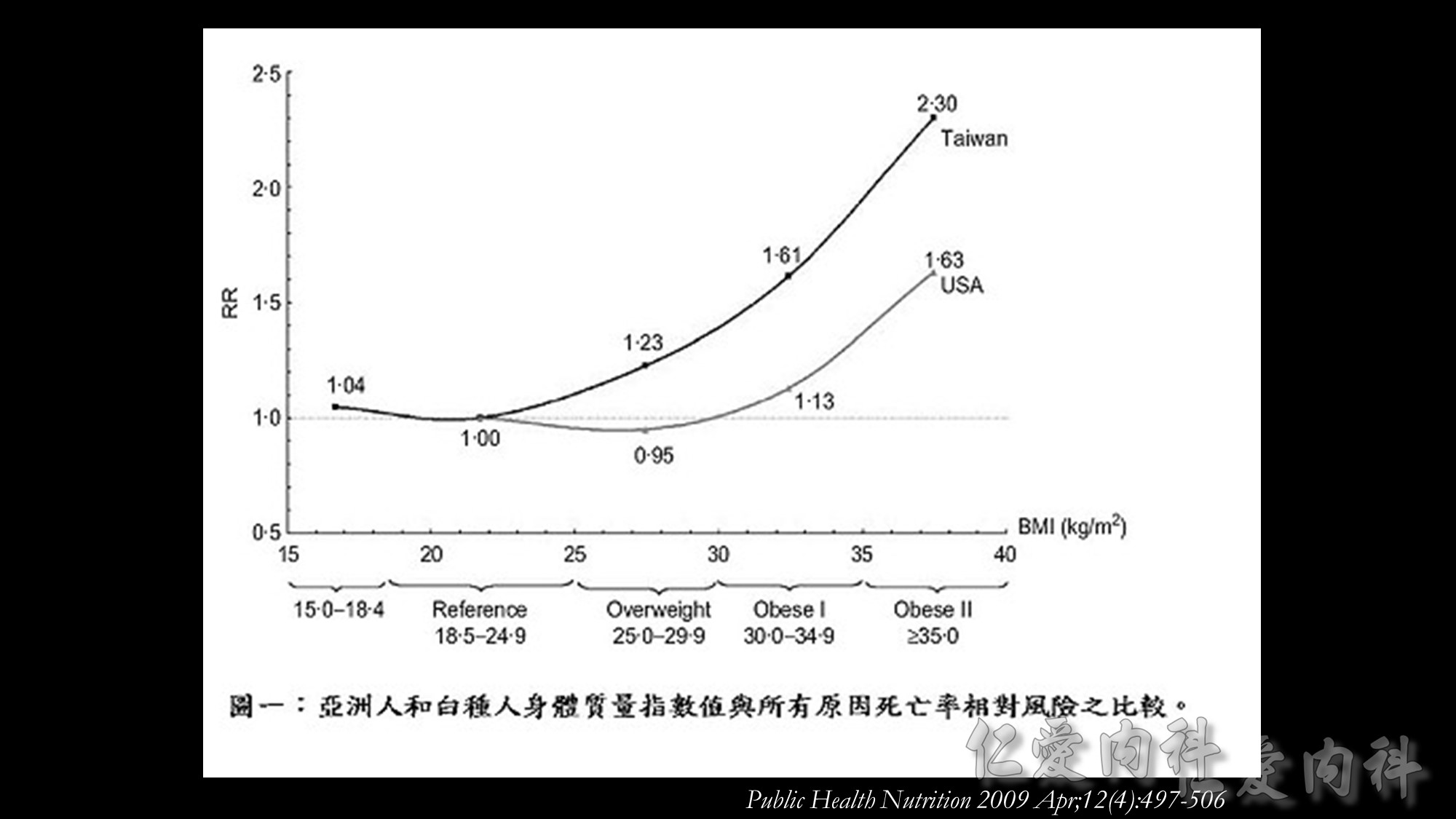
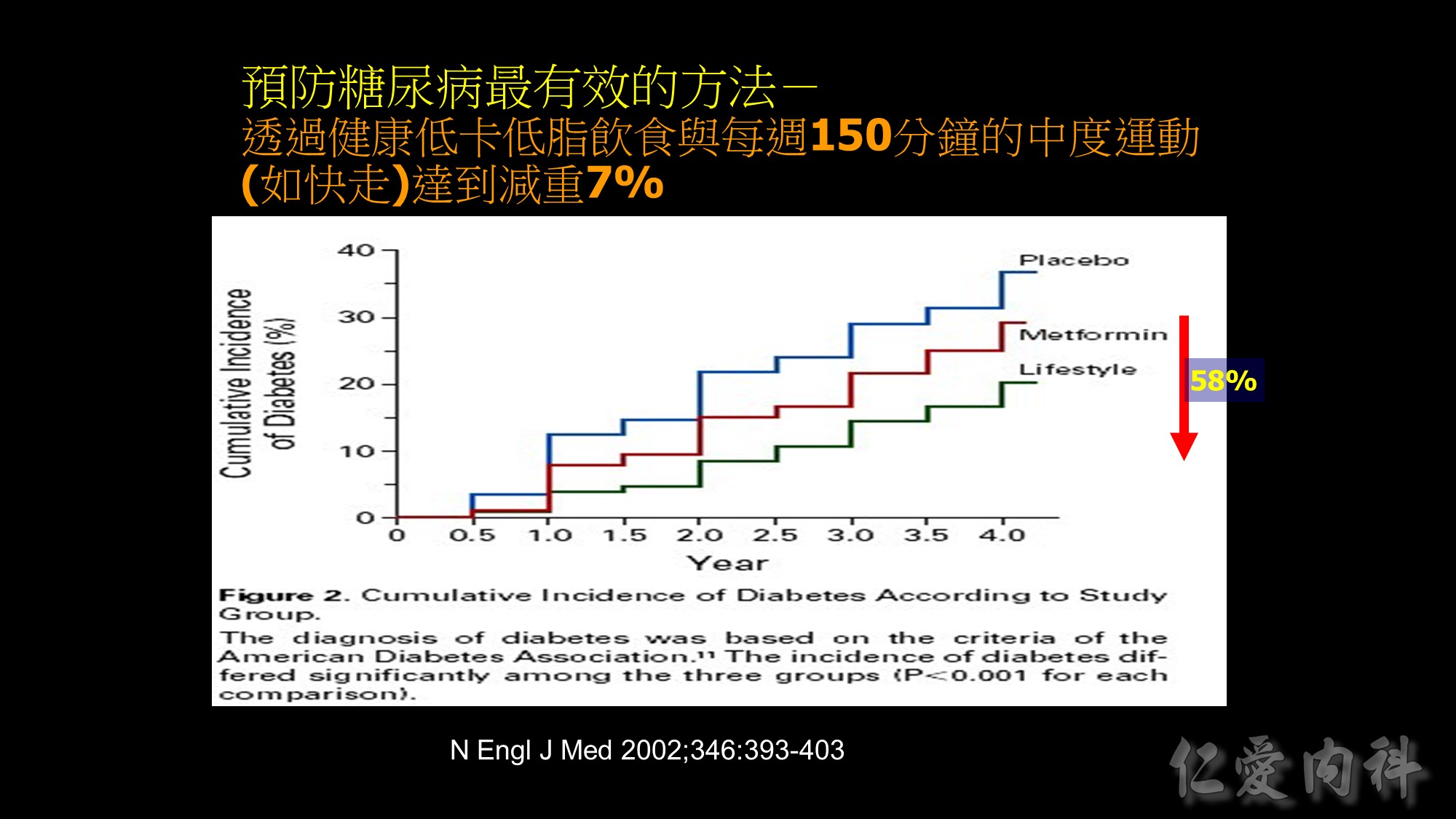
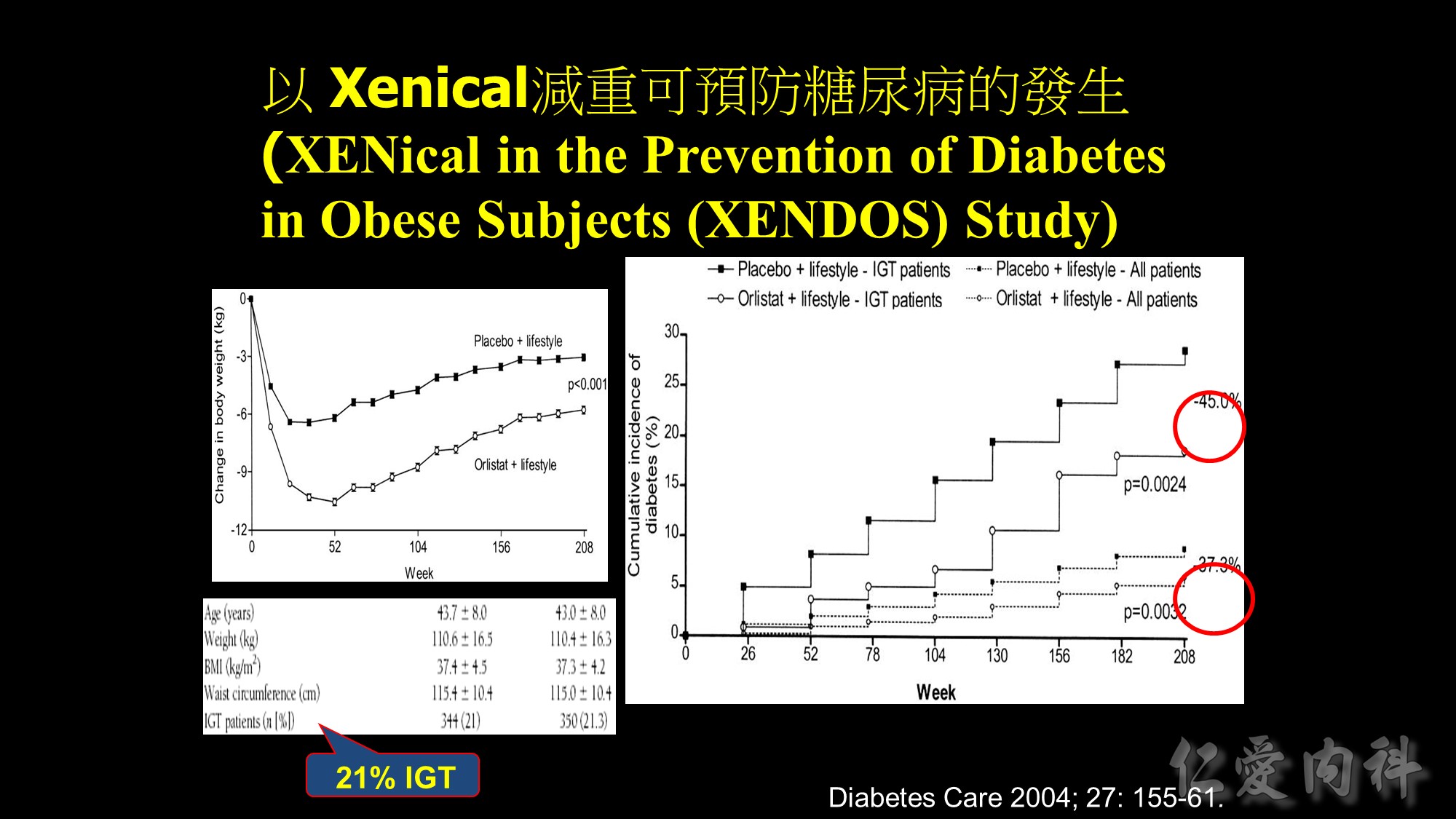
國衛院群體健康科學研究所衛生政策研究組溫啟邦研究員之研究團隊,利用世代研究(cohort study)探討當同時處於相同BMI值時,亞洲人種的死亡率是否比白種人高,且BMI為何數值時死亡率開始增加,藉此評估WPRO所建議的體重過重與肥胖之切點是否適用於亞洲人種。
此研究的族群為1989至1992年參加健康檢查40歲或以上的36,386名台灣民眾,分為吸菸組與非吸菸組,在校正過年齡、性別、吸菸習慣、飲酒習慣及身體活動後,以Cox比例風險模型計算WHO與WPRO所界定的BMI過重基準值的相對危險性(relative risk)。
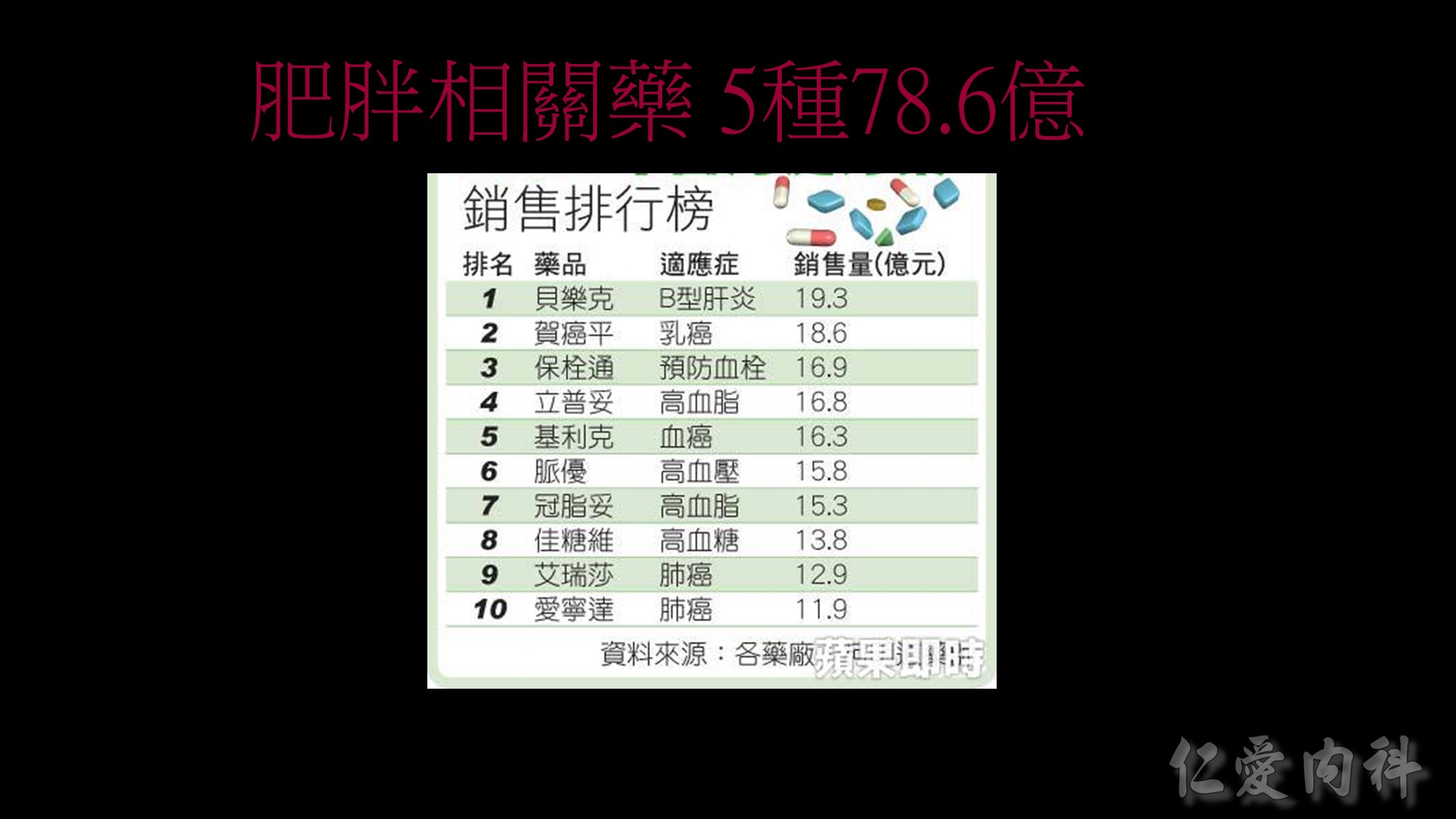
體重過重與肥胖盛行率
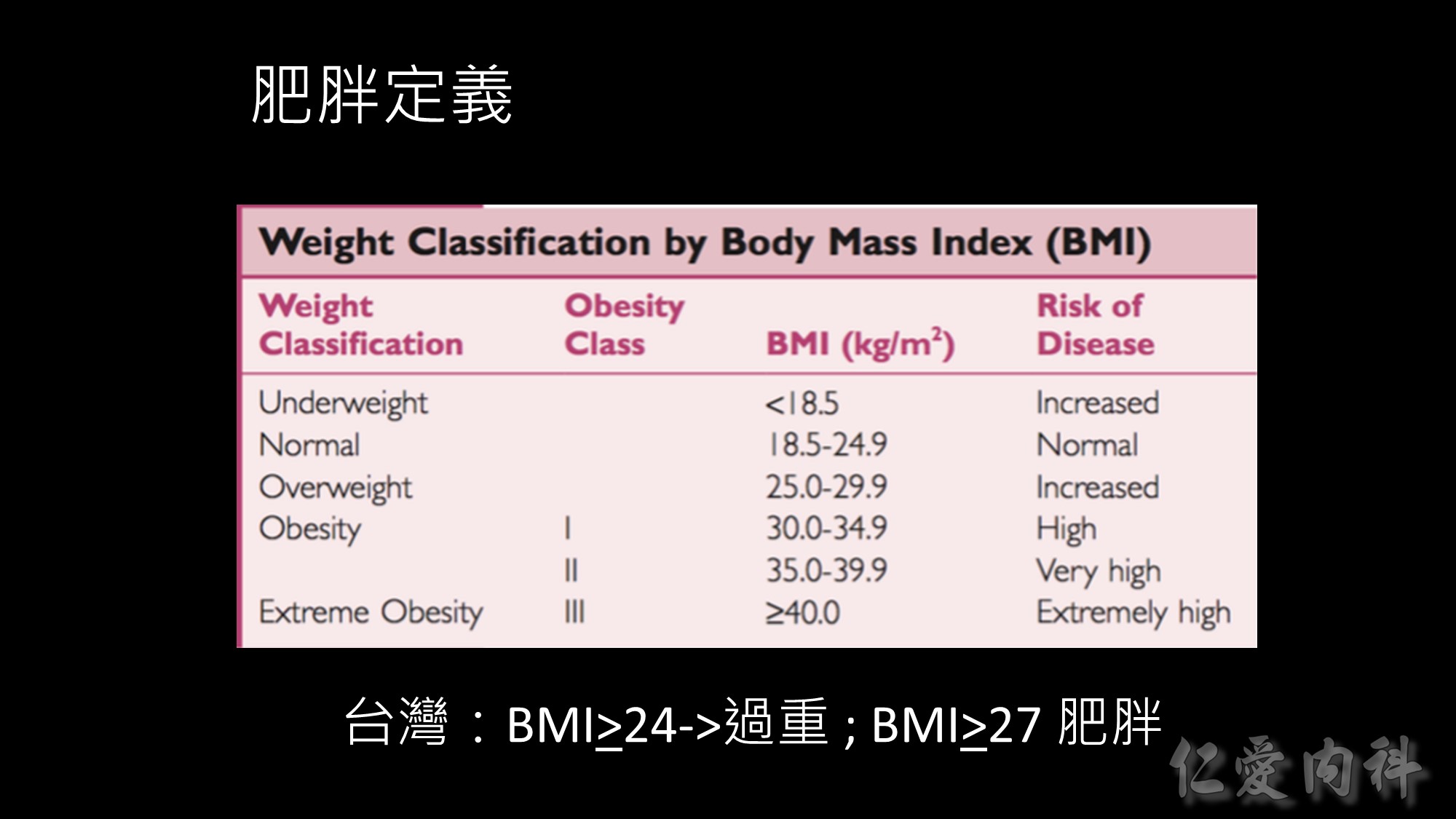
依據WHO的定義,台灣成人體重過重與肥胖盛行率分別為23%及4%,但如依據WPRO定義,台灣成人體重過重與肥胖盛行率分別為20.8%及27.1%,肥胖盛行率是世界衛生組織定義的7倍。此外,台灣成人體重過重與肥胖的比例,隨著年齡增加在50至59歲達到高峰。BMI值與死亡風險
此研究發現,依據WHO定義的理想體重範圍(BMI=18.5-24.9 kg/m2 ),台灣民眾的全死因死亡率(all-cause mortality)風險會隨著BMI增加,體重過重與肥胖群之相對危險性顯著增加。此研究也應用美國國家衛生及營養調查(U.S. National Health and Nutrition Examination Survey,NHANES)資料進行比較,發現當台灣民眾的BMI值為25.0-29.9 kg/m2 時,其死亡率相對風險與美國民眾BMI值為30.0-34.9 kg/m2 者相符,台灣民眾BMI值為30.0-34.9 kg/m2 時的死亡率相對風險與美國民眾BMI值為35.0-39.9 kg/m2 者相似如果以WPRO之定義為標準,當BMI值=18.5-22.9 kg/m2 時,台灣民眾的所有原因死亡率風險並未增加,但當BMI值超過25時,數值每增加1單位,相對所有原因死亡率風險(relative mortality risk from all causes)便增加9%。以國際疾病分類(ICD-9)項目進行之病因分析結果發現,因肥胖而導致之額外死亡佔台灣所有死因的8.6%。
溫啟邦博士研究團隊認為如果台灣民眾可以代表亞洲人種,這項研究的數據證明,要重新定義亞洲人種的BMI肥胖切點。體重過重的台灣民眾比美國體重過重的民眾,擁有明顯又更高的所有原因死亡率風險。亞洲族群的體脂肪比例比白種人多、且擁有更高的心血管疾病風險,而這些現象並不只限於肥胖者,在體重過重者已有此現象。如果下修WHO對BMI肥胖定義之切點(從30.0 kg/m2 改為25.0 kg/m2 ),此舉才能實際呼應WHO訂定BMI體重標準的用意:指出族群中具有危害健康的危險因子且需公共衛生或臨床介入者。
Source: 國家衛生研究院電子報
馬偕醫院發表一份為期10年(1993-2003年)之大型研究,研究發現身體質量指數(BMI)超過23時,罹患高血壓與高尿酸血症的比率,分別為正常人的2.4倍與1.8倍,因此建議衛生署應下修過重與肥胖標準。
Source: 童綜合醫療社團法人童綜合醫院
2000年世界衛生組織,在缺乏亞洲肥胖危險性相關資料下,曾暫時建議分別以BMI 23與25作為亞洲人過重與肥胖的切點,並鼓勵亞洲各國積極進行資料收集與分析工作,以作為日後訂定亞洲人肥胖定義之參考。而我國則在民國91年(2002年),由本署邀集國內專家學者成立「肥胖定義及處理委員會」,參考我國國民營養變遷調查結果、國人代謝症候群相關研究,訂定我國之肥胖標準及處理方法,以提供公共衛生、醫界及其他健康相關行業作為肥胖預防、篩選、及介入之參考。
由於研究資料顯示,BMI24以上成人中,有68%男性、65%女性有代謝症候群相關病徵;BMI24以下的成人中,有68%男性、70%女性無此病徵;而BMI27以上成人85%以上有肥胖相關的代謝症疾病。因此,「肥胖定義及處理委員會」之「成人肥胖定義小組」建議將國人過重與肥胖的切點定為BMI 24與27。
Source: 國際厚生健康園區

The strikingly positive association between low BMI and death from causes other than cardiovascular disease or cancer was primarily the result of deaths due to respiratory diseases. After exclusion of deaths from respiratory diseases, the positive association with low BMI was substantially reduced. It is possible that the strong association observed between low BMI and death from respiratory diseases could be explained, in part, by reverse causation, since respiratory disease can lead to weight loss long before the clinical diagnosis is made.
In the cohorts of East Asians, including Chinese, Japanese, and Koreans, the lowest risk of death was seen among persons with a BMI in the range of 22.6 to 27.5.
The authors also make a conclusion that overall, the risk of death among Asians, as compared with Europeans, seems to be more strongly affected by a low BMI than by a high BMI. Given the limitations of the current study, in which the risk of death was used as the outcome, additional studies are needed to quantify the association between BMI and the incidence of disease, in order to better define BMI criteria for overweight and obesity in Asians.
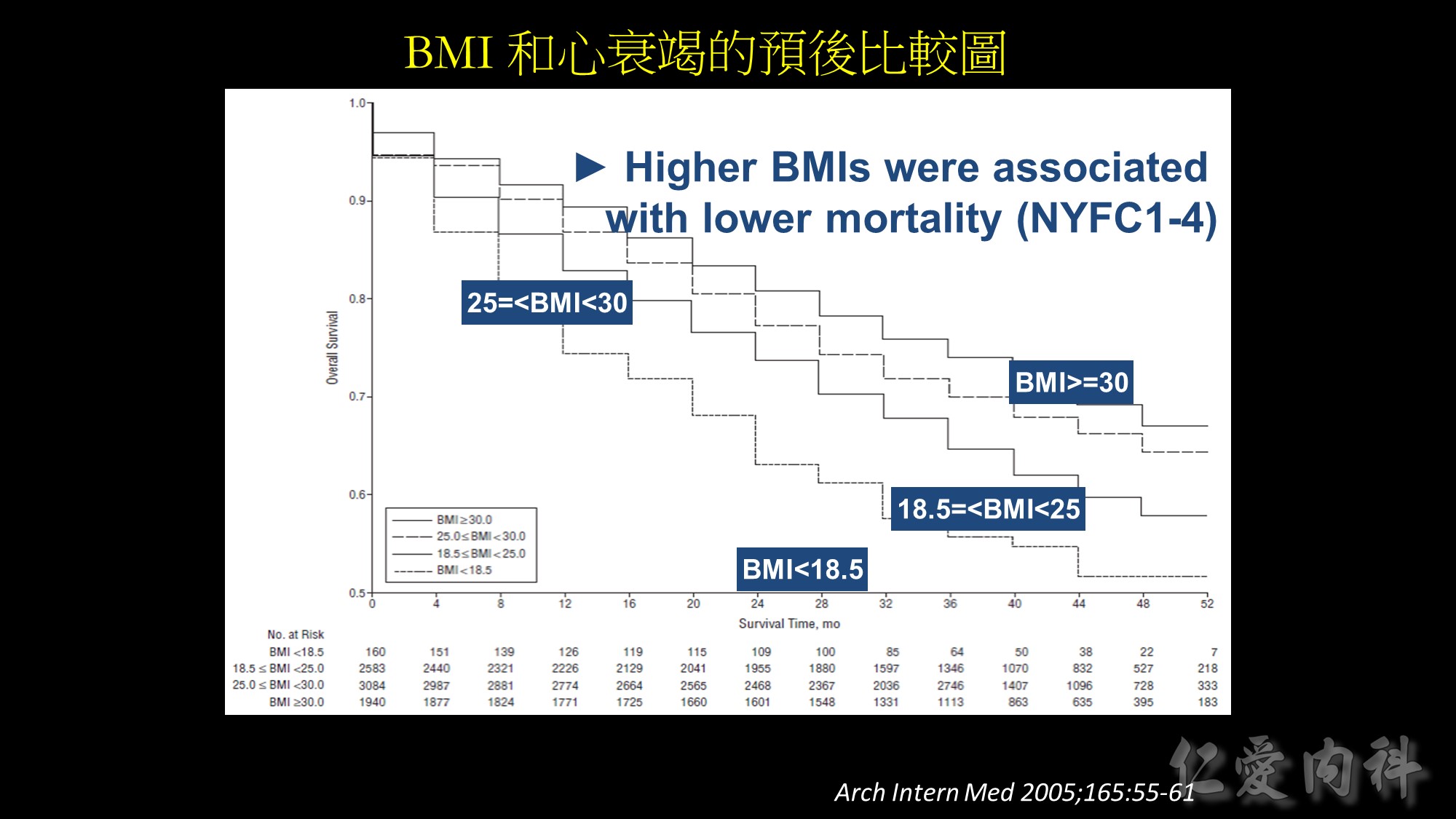
Background: In the general population, obesity is associated with increased risk of adverse outcomes. However,
studies of patients with chronic disease suggest that overweight and obese patients may paradoxically have better outcomes than lean patients. We sought to examine the association of body mass index (BMI) and outcomes in stable outpatients with heart failure (HF).
Methods: We analyzed data from 7767 patients with stable HF enrolled in the Digitalis Investigation Group trial. Patients were categorized using baseline BMI (calculated as weight in kilograms divided by the square of height in meters) as underweight (BMI 18.5), healthy weight (BMI, 18.5-24.9, overweight (BMI, 25.0-29.9), and obese (BMI 30.0). Risks associated with BMI groups were evaluated using multivariable Cox proportional hazards models over a mean follow-up of 37 months.
Results: Crude all-cause mortality rates decreased in a near linear fashion across successively higher BMI groups, from 45.0% in the underweight group to 28.4% in the obese group (P for trend.001). After multivariable adjustment,overweight and obese patients were at lower risk for death (hazard ratio [HR], 0.88; 95% confidence interval [CI], 0.80-0.96, and HR, 0.81; 95% CI, 0.72- 0.92, respectively), compared with patients at a healthy weight (referent). In contrast, underweight patients with stable HF were at increased risk for death (HR 1.21; 95% CI, 0.95-1.53).
Conclusions: In a cohort of outpatients with established HF, higher BMIs were associated with lower mortality risks; overweight and obese patients had lower risk
of death compared with those at a healthy weight. Understanding the mechanisms and impact of the “obesity paradox” in patients with HF is necessary before recommendations are made concerning weight and weight control in this population.
Source: Arch Intern Med. 2005;165:55-61

There are several methods of measuring abdominal visceral fatness, which is well known to correlate with cardiometabolic risk. The gold standard is CT or MRI. Sonography is a non-invasive tool and the correlation coefficient is 0.799. In contrast to conventional BIA, Omron and Tanita companies developed new BIA method to measure the abdominal body fat with moderate to good correlation with gold standard. The latest development of abdominal fat measurement is GE Health Lunar iDXA, and it has highest correlation=0.957. In the future, these tools may provide more evidences to demonstrate the relationship between abdominal fatness and cardiometabolic diseases in subjects with obesity paradox.


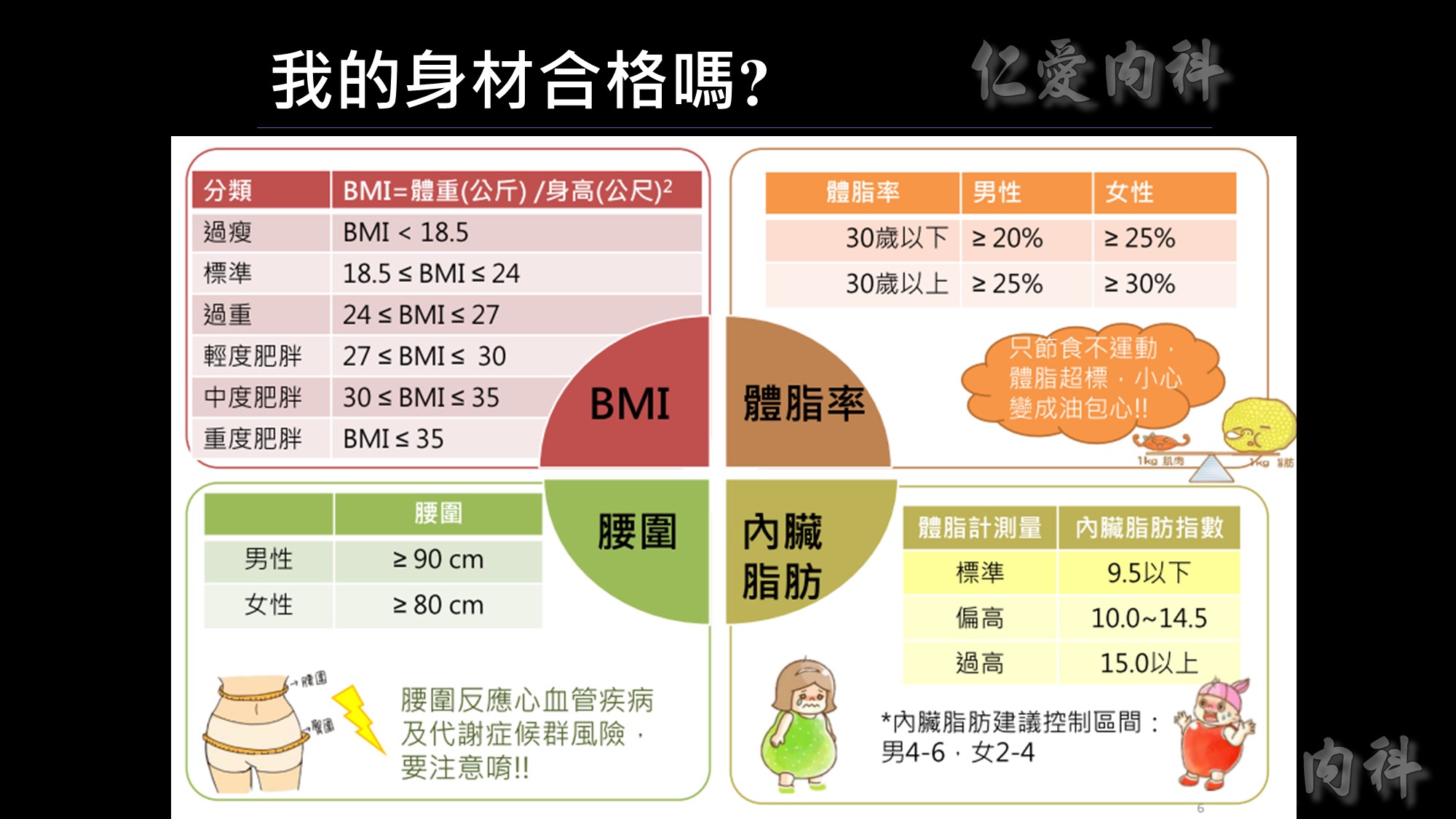
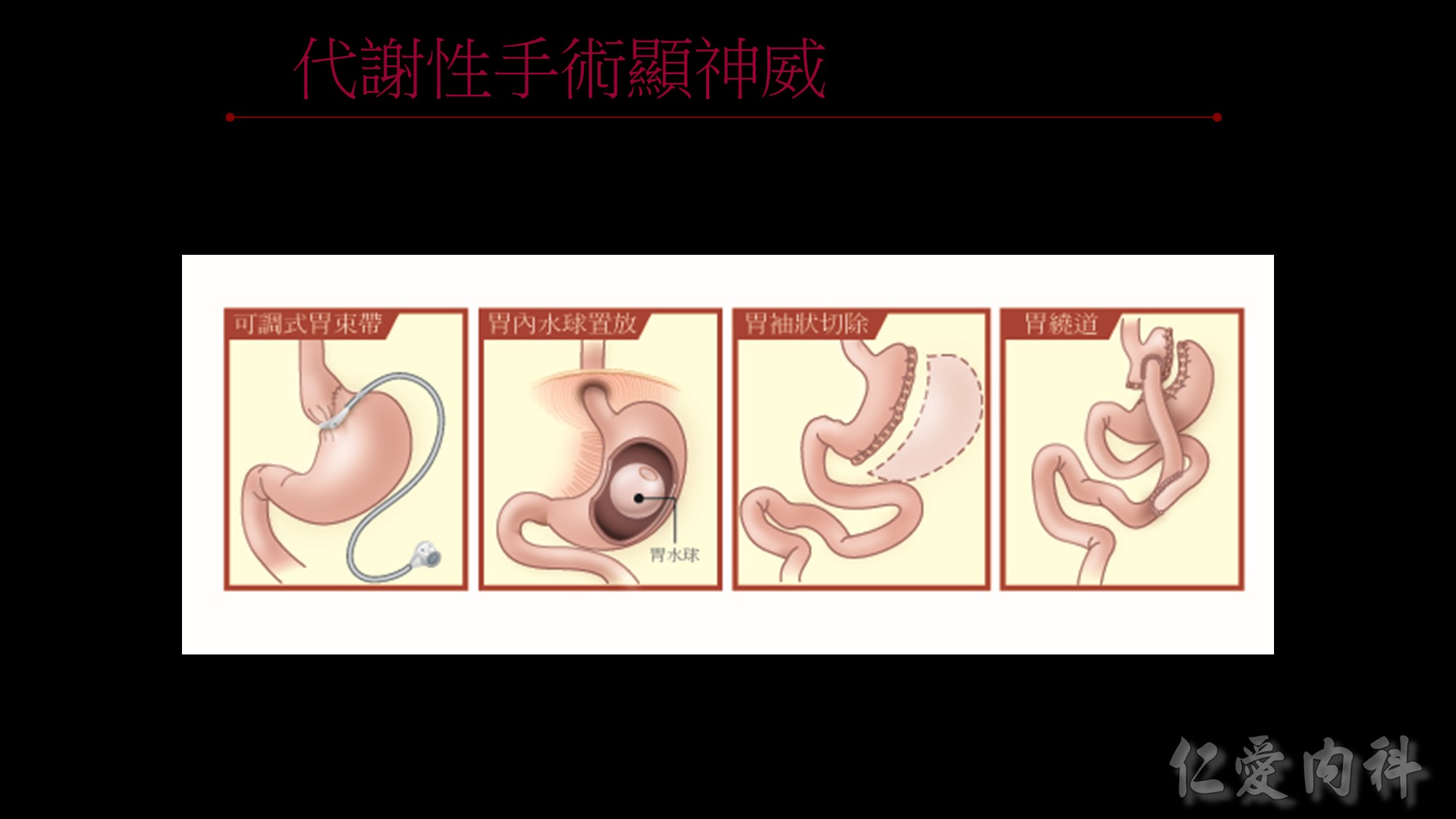
為何說不能僅依BMI來界定是否肥胖呢?我們看看這張圖~
體重、身高相同、BMI相同但脂肪、腰圍的比例卻大不相同。
所以監測肥胖的四大指標為BMI、體脂率、腰圍、內臟脂肪!
也越來越多研究顯示,肥胖和三高及慢性疾病都有很大的關係; 甚至是現在很常提到的代謝症侯群及不孕症、癌症等疾病都和肥胖相關。
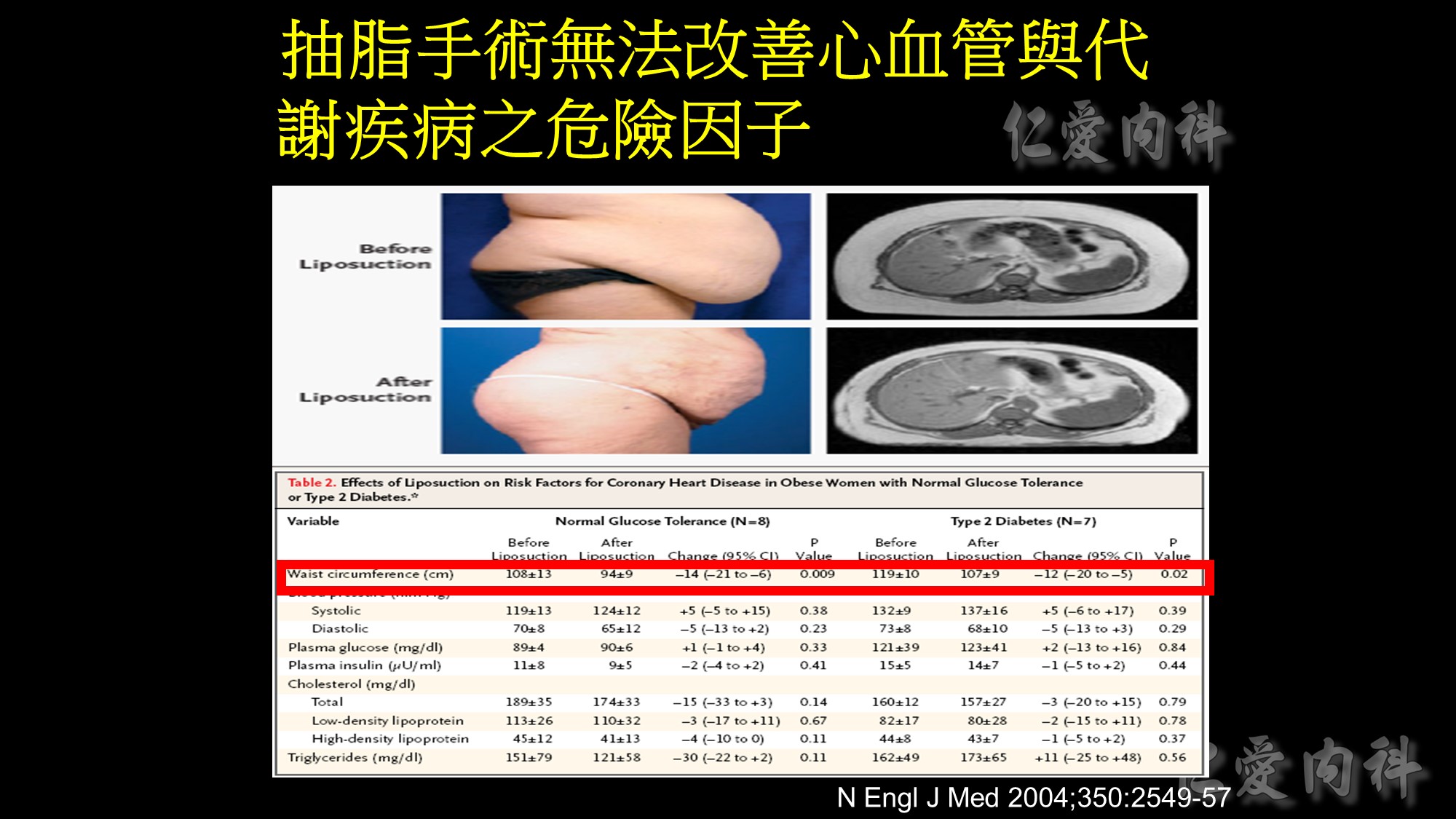
體重包括了肌肉、脂肪、骨骼、水分等等成份的重量,我們其實更應該關心脂肪的多寡,因為脂肪(尤其內臟脂肪)的囤積與許多代謝疾病有關,例如肥胖、代謝症候群、糖尿病、高血壓、高血脂、脂肪肝、心血管疾病等,單看體重,無法得知脂肪的多寡。例如對於長期運動健身的人而言,身體充滿肌肉,肌肉的密度比脂肪來得高,因此這些人的體重往往不低,甚至單看體重會以為是個胖子,但其實他們的外型看來並不胖,而是結實精壯。
相反地,許多體重在標準範圍內的人,實際上體脂肪是超標的,這些人因為體脂肪高,身材較為鬆軟,俗稱泡芙族,或者我稱之為偷肥族 (取自TOFI諧音. Thin-Outside-Fat-Inside, 外型看來正常,但內部是肥胖的),若只看體重,會以為自己健康,但其實這些人是有健康風險的,甚至認為體重標準而肆無忌憚亂吃、作息不正常而更讓自己身陷疾病風險。由此更顯現出只看體重或BMI是不夠的。


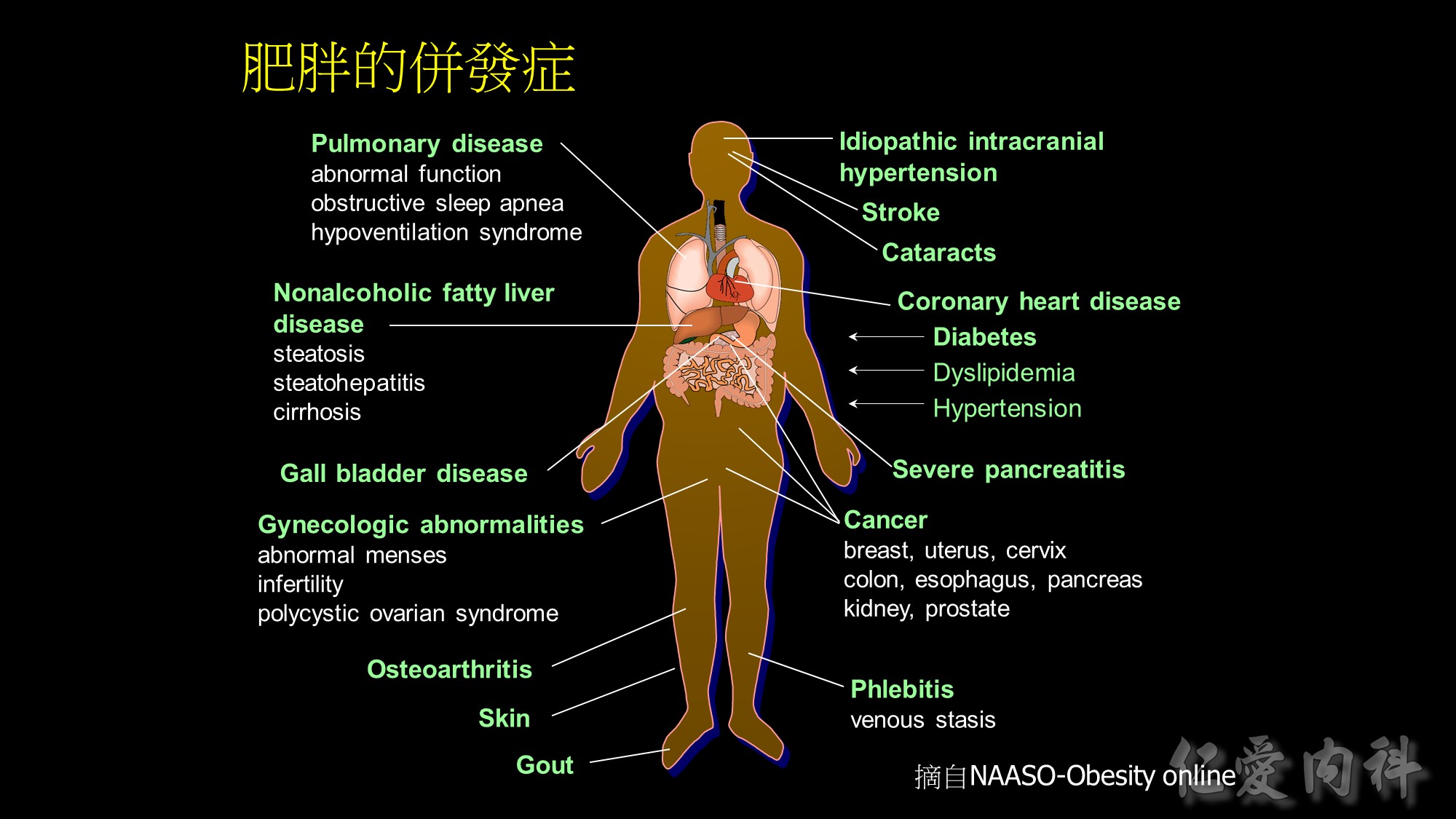
Medical complications of obesity
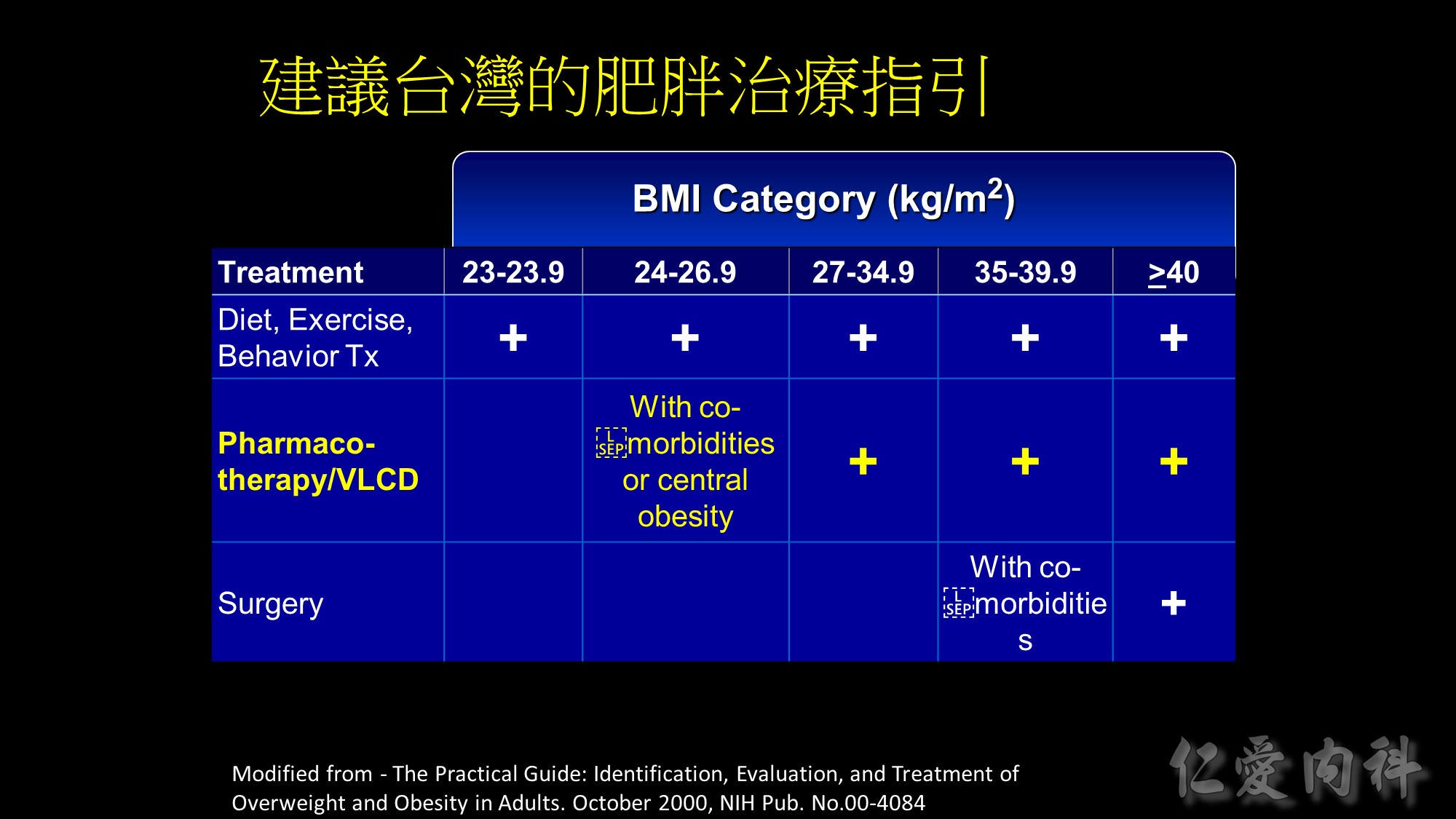
Guide for selecting obesity treatment
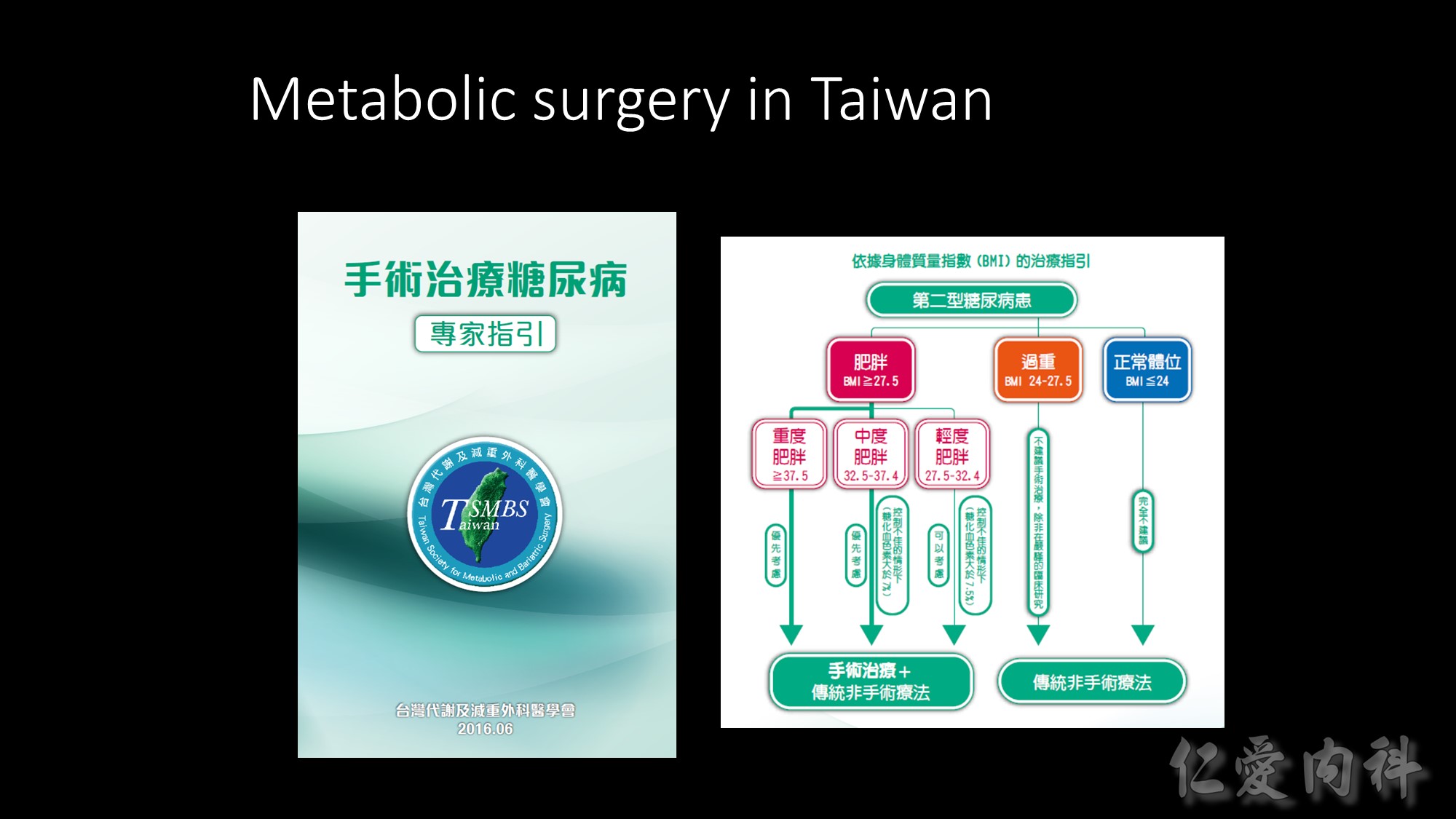
This table summarizes the guidelines for selecting treatment options for obesity. Any effective treatment plan must consider the patient’s willingness to undergo therapy, his/her ability to comply with specific treatment approaches, access to skilled caregivers, and financial considerations. Lifestyle modification, which involves a program of appropriate diet, physical activity, and behavior therapy, should be considered for all patients with a body mass index (BMI) ³25 kg/m2. Long-term pharmacotherapy should be considered in appropriate patients who were unable to achieve adequate weight loss after 6 months of lifestyle therapy and who have a BMI ³30 kg/m2, or ³27 kg/m2 with concomitant obesity-related disease. Bariatric surgery may be necessary in patients with severe obesity who failed to lose weight with non-surgical therapy. Eligible surgical candidates should have a BMI ³40 kg/m2 or a BMI ³35 kg/m2 and a concomitant serious obesity-related disease.
Source: The Practical Guide: Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. October 2000, NIH Pub No 00-4084.
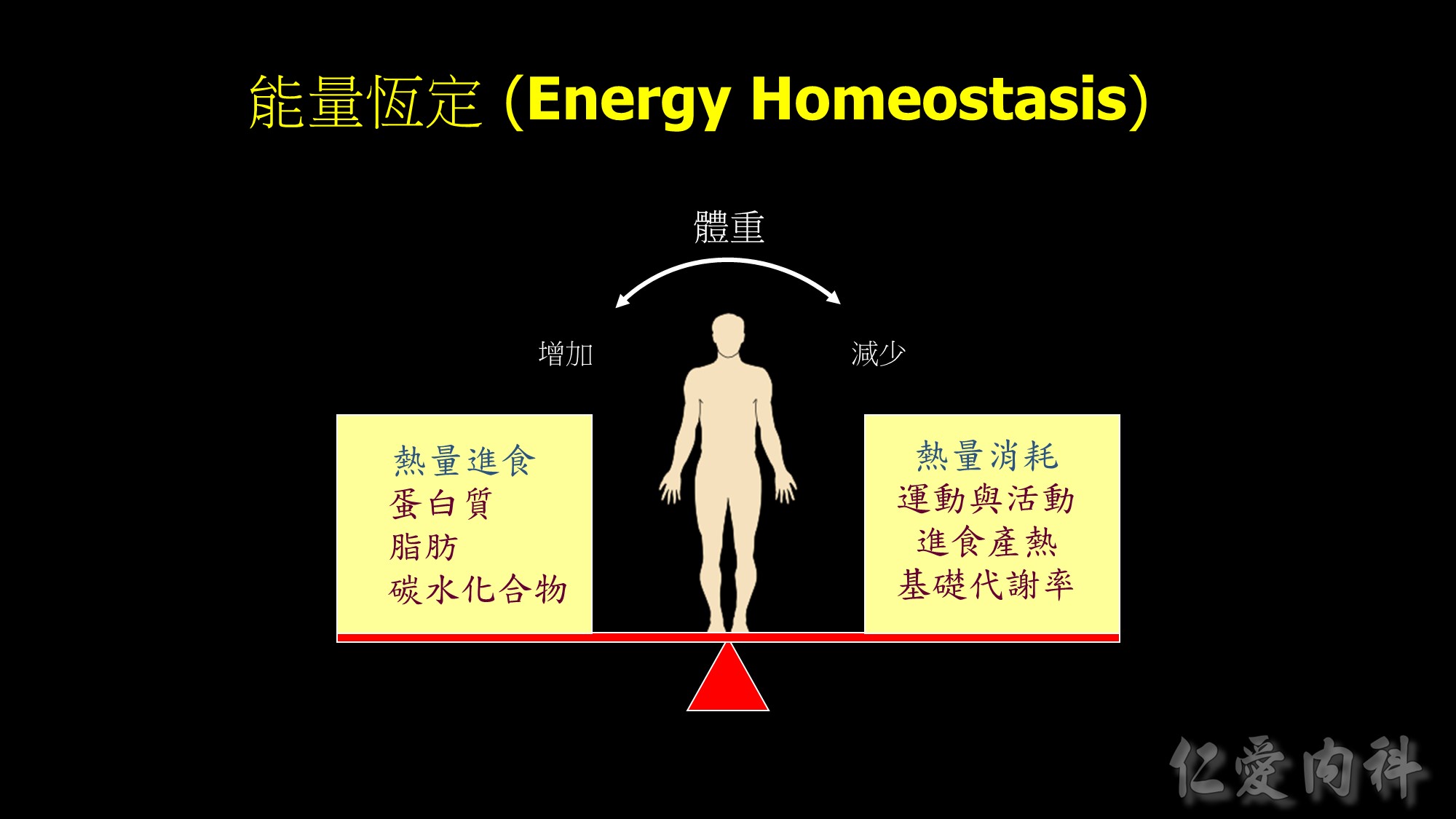
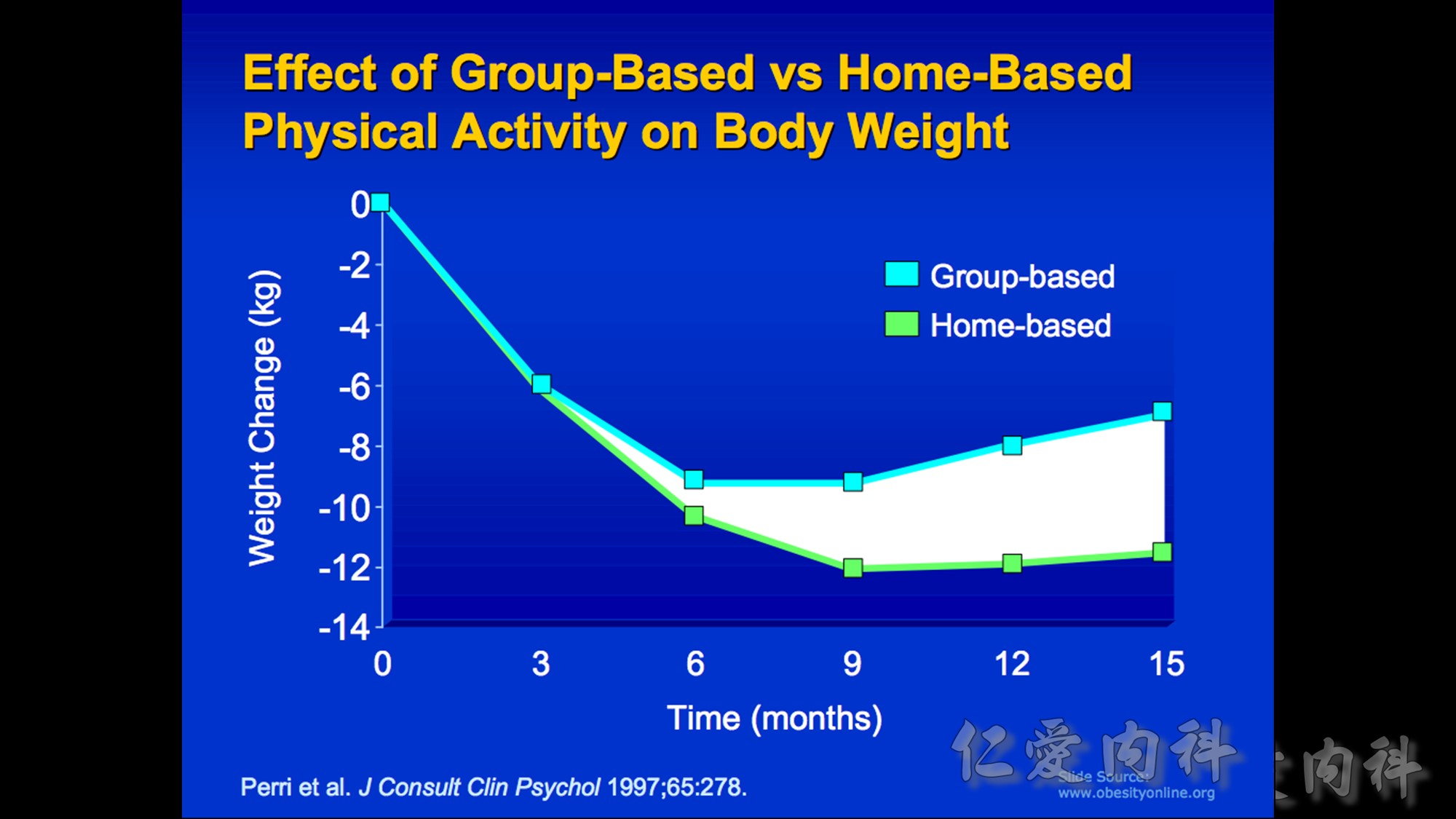
Body weight maintained through complex mechanisms ensuring a constant supply of energy for cellular functions.



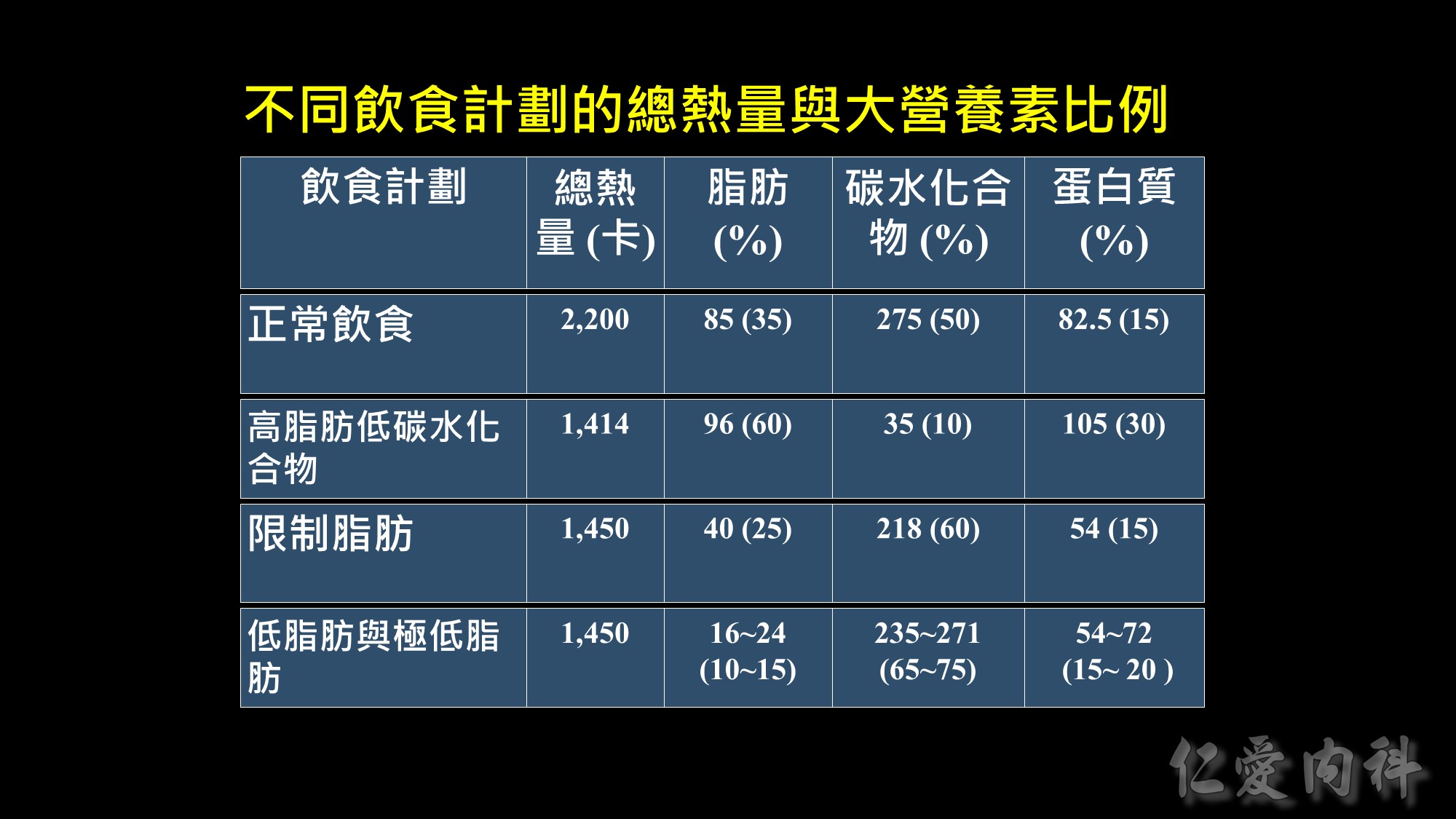
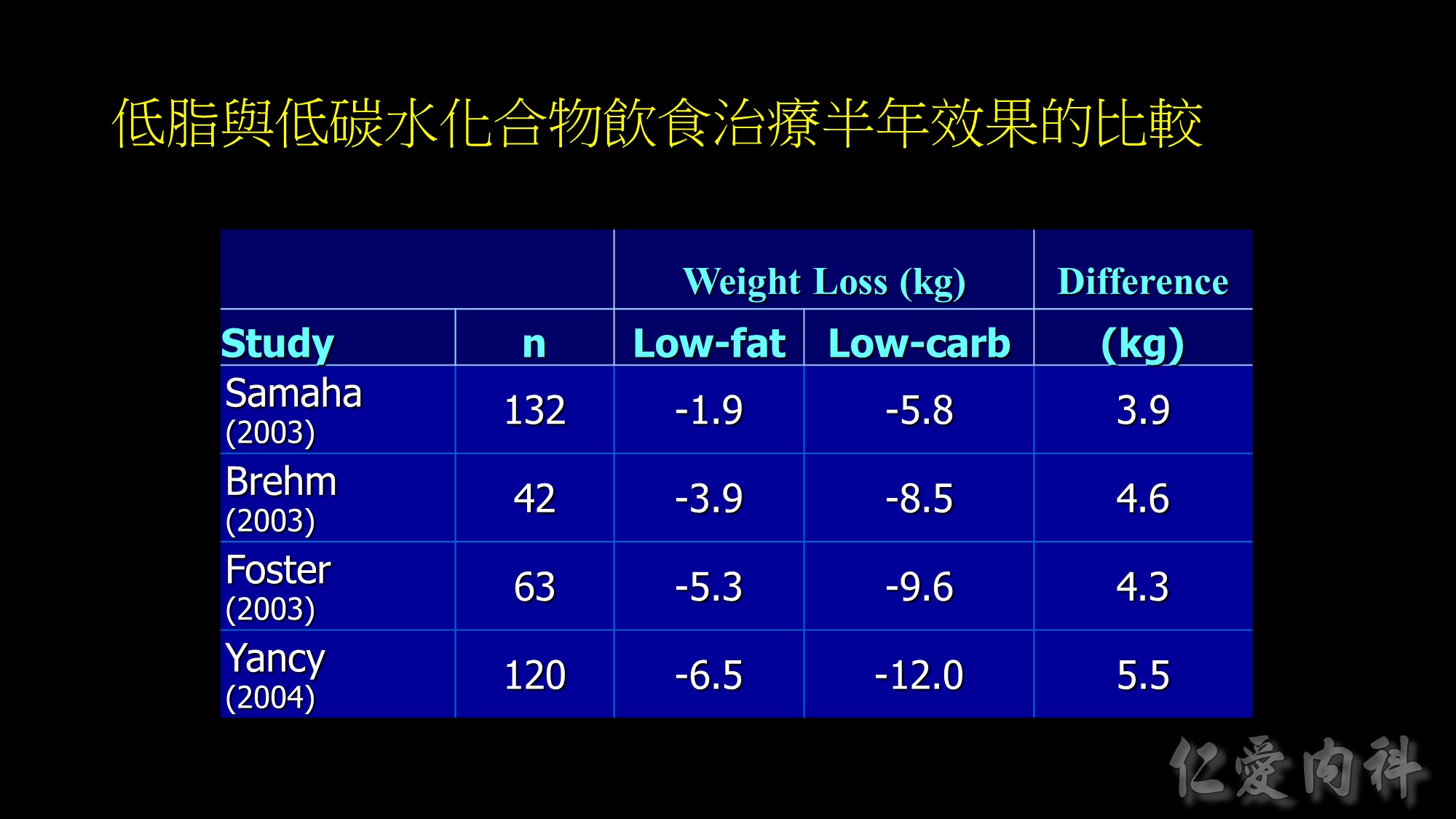
Weight loss at 6-months in RCTs of low-fat vs low-carbohydrate diets
Recently, there has been increased interest in the use of low-carbohydrate diets as potential therapy for obesity. The results of five RCTs in adults [1-5] found that subjects randomized to a low-carbohydrate, high protein/high fat diet (approximately 25-40% carbohydrate) achieved greater short-term (6 months) [1-4], but not long-term (12 months) [3, 5], weight loss than those randomized to a low-fat diet (approximately 25-30% fat, 55-60% carbohydrate). A consistent difference in weight loss at 6 months was observed between groups across studies; subjects randomized to the low-carbohydrate diet lost 4-5 kg more weight than those randomized to the low-fat diet. The data from these studies also found greater improvements in serum triglyceride and HDL-cholesterol concentrations, but not in serum LDL-cholesterol concentration, in the low-carbohydrate group than in the low-fat group. In addition, glycemic control was better with low-carbohydrate than a low-fat diet therapy in subjects who had type 2 diabetes [1, 5].
1.Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams T, Williams M, Gracely EJ, Stern L: A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med 348:2074-2081, 2003.
2.Brehm BJ, Seeley RJ, Daniels SR, D’Alessio DA: A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol.Metab 88:1617-1623, 2003
3.Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S: A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med 348:2082-2090, 2003.
4.Yancy WS, Olsen MK, Guyton JR, et al. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia. Ann Intern Med 2004;140:769-777.
5. Stern L, Iqbal N, Seshadri P, et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow up of a randomized trial. Ann Intern Med 2004;140:778-785.




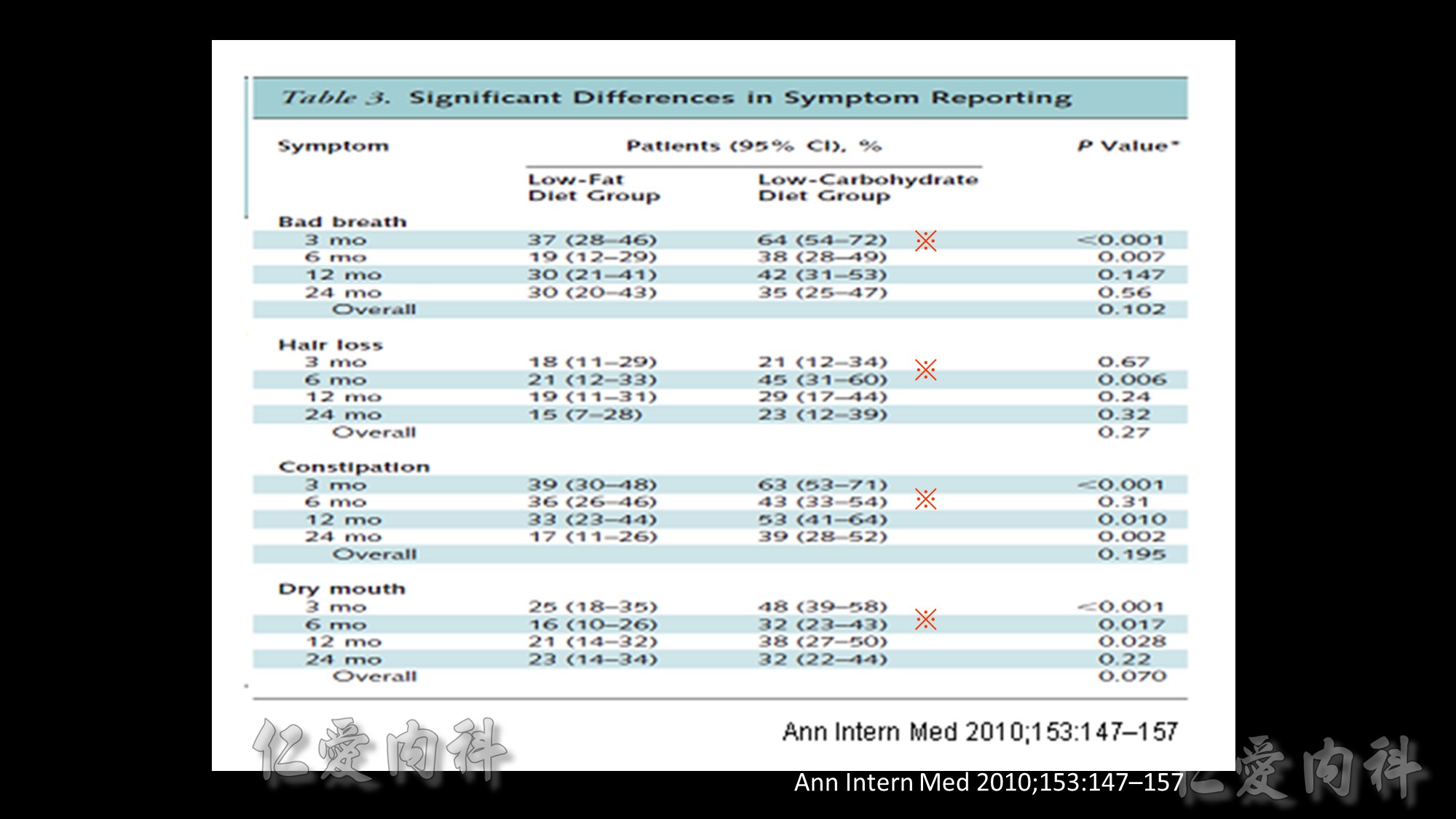









在講效果前我們先講一下FDA在核准藥品的必要條件:

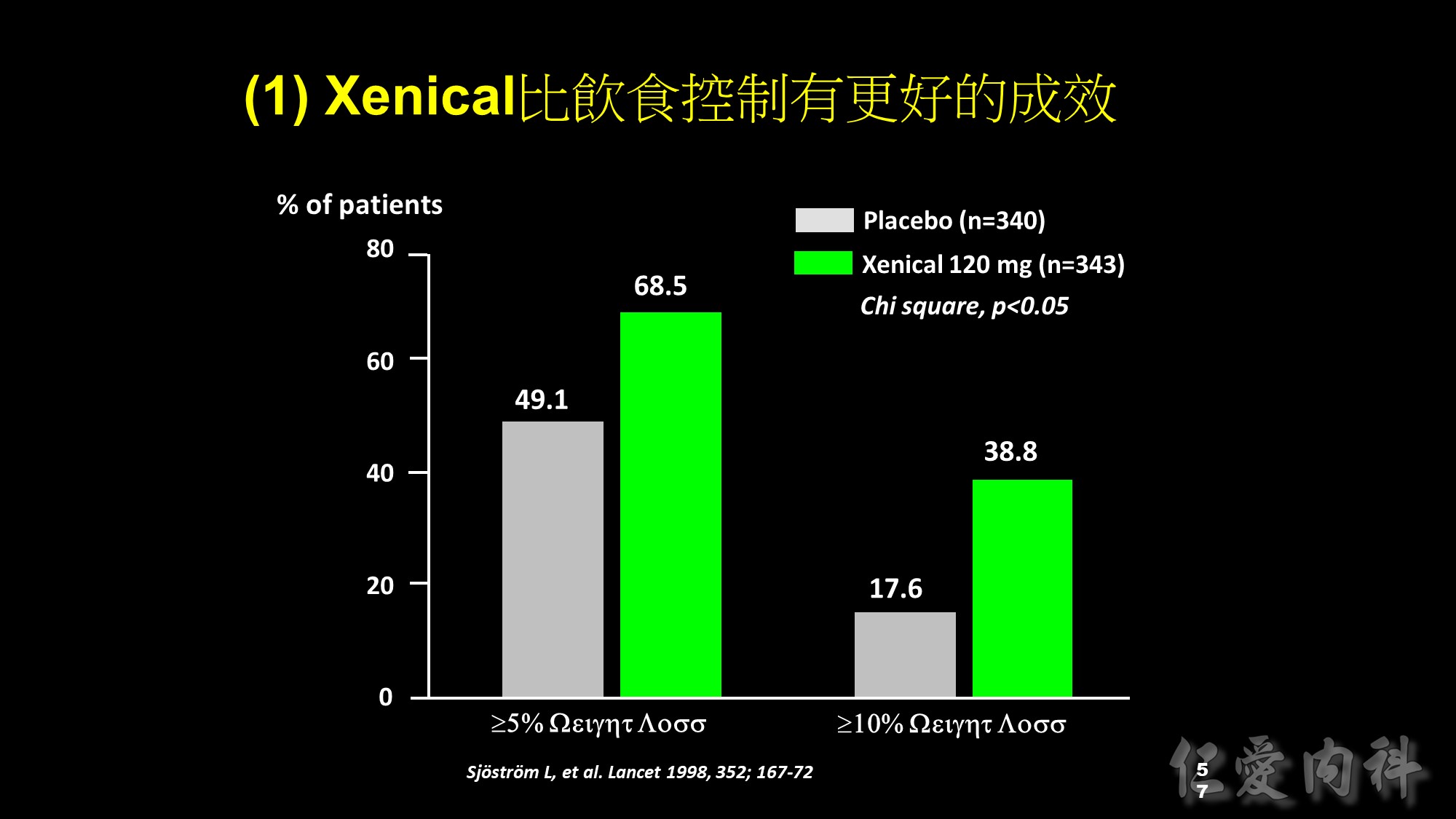
Slide 14: Categorical analysis of weight loss
As well as a greater mean reduction in body weight, more Xenical-treated patients lost large amounts of weight compared with patients receiving placebo. After 1 year of treatment, 68.5% of patients in the Xenical group lost >5% of their initial body weight, compared with only 49.1% in the placebo group (p<0.001). In addition, the percentage of patients achieving weight loss of >10% was significantly greater in the Xenical-treated patients (38.8% vs 17.6%; p<0.001).

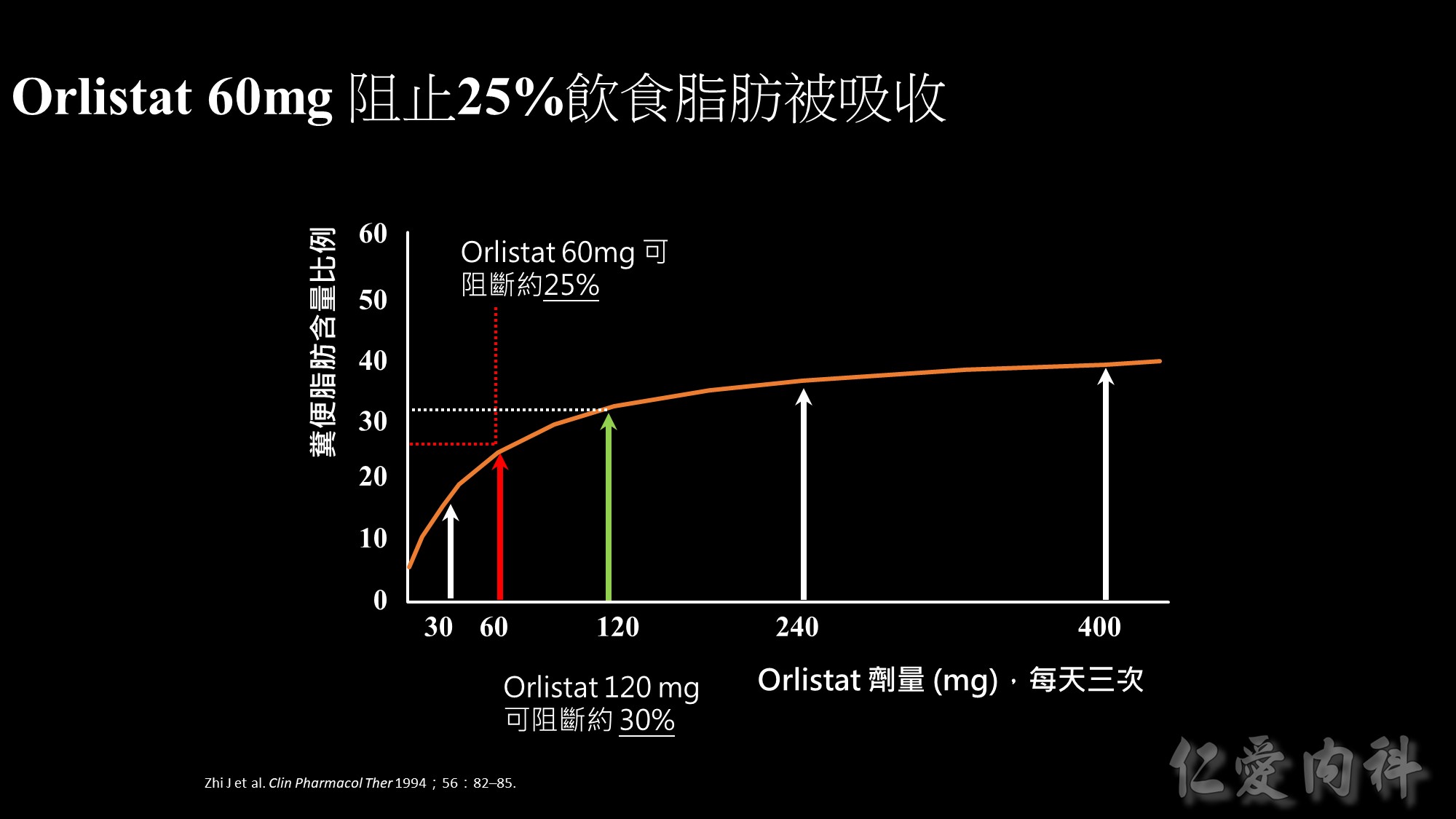
This graph illustrates the effect of orlistat at doses ranging from 30 to 400 mg taken three times daily (tid) – as measured by how much fat is being excreted.
Orlistat 60 mg tid blocks the absorption of about 25% of dietary fat. Orlistat 120 mg tid (double the dose) blocks the absorption of about 30% of dietary fat.
For fat excretion,60 mg provides approximately 80% of the efficacy of the 120 mg dose.
After an initial steep rise,the graph tends towards a plateau at doses >120 mg.
Overall,these results tell us that there would be little improvement in efficacy if someone was to exceed the recommended dose (60 mg tid).


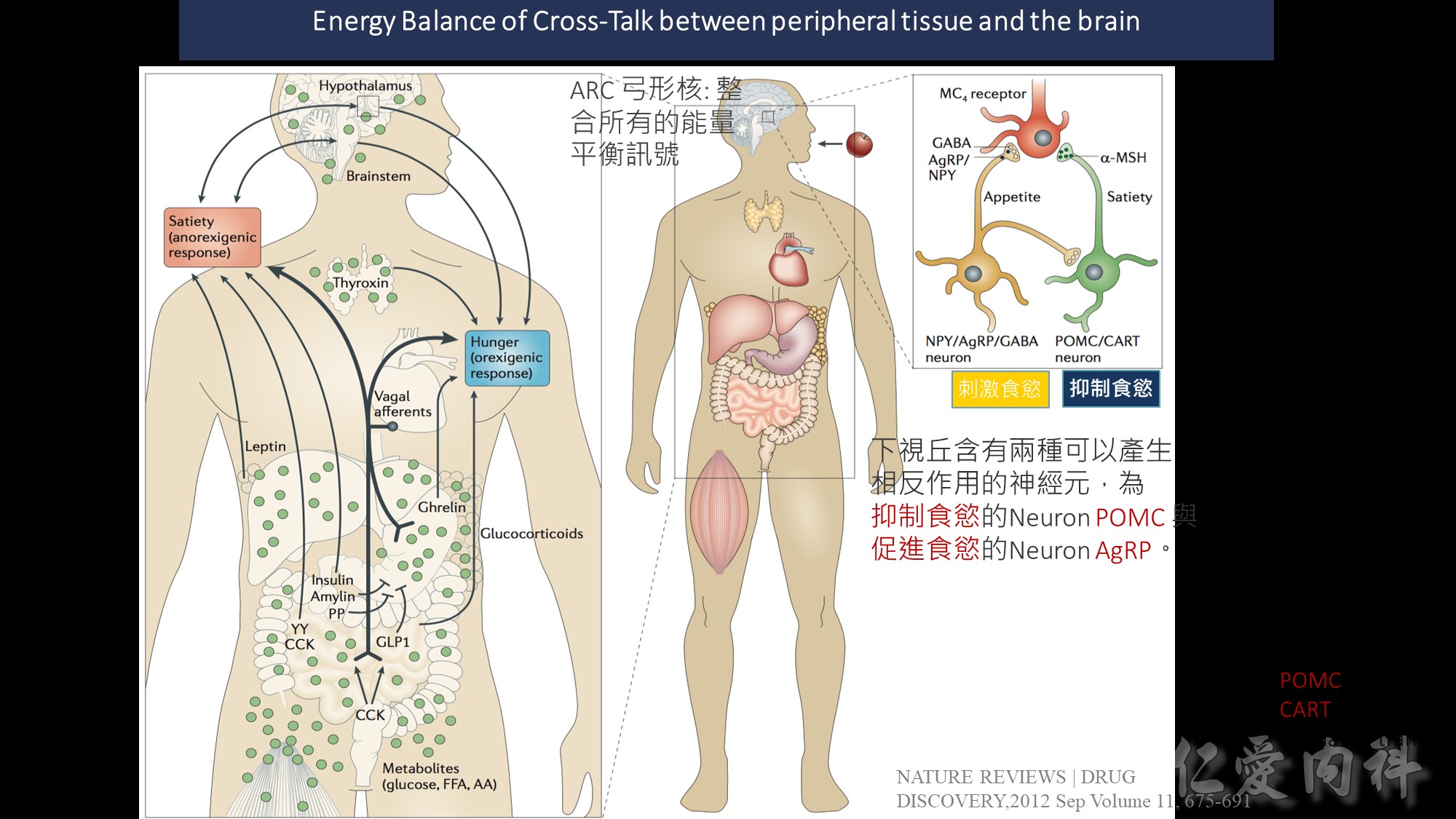
Peripheral factors indicative of long-term energy status are produced by adipose tissue (leptin 瘦素 , adiponectin脂聯素 ) and the pancreas 胰臟 (insulin) 胰島素
Acute hunger signal : ghrelin 飢餓素
Satiety signals 飽足感訊息傳遞 such as the gut hormones peptide YY (PYY), pancreatic polypeptide (PP), amylin and oxyntomodulin (OXM) indicate near-term energy status
ARC 弓形核: 在下視丘,位置在第三腦室和median eminence(中值隆起)之間,負責整合所有的能量平衡訊號。
ARC透過(1) median eminence的半透性微血管接受化學性訊號,並接受(2)來自NTS的神經訊號
inhibit feeding. : Anorexigenic cocaine- and amphetamine – regulated transcript (CART) and
pro-opiomelanocortin (POMC) neurotransmitters, both of which inhibit feeding.

脂肪激素與瘦體素
瘦體素是一種含有167個胺基酸的蛋白質,主要由脂肪組織所分泌。脂肪組織的作用之一是貯存能量,因此瘦體素的濃度高低主要反應生物體內的能量貯存狀況。一般人血中瘦體素濃度和體脂肪多寡成正比,愈胖的人血中瘦體素濃度愈高,反之則低。當瘦體素濃度降低時,會刺激中樞神經系統增加食慾,減少能量消耗,當瘦體素濃度增加時,會減少食慾。
Source: 台中榮民總醫院
集中投射神經元
集中投射神經元包含神經肽Y(neuropeptide Y,NPY)、刺鼠肽基因相關蛋白(agouti-related protein,AgRP)和抑制性神經遞質γ-氨基丁酸(GABA)。集中投射神經元在細胞核的最腹內側部(ventromedial),突出至下視丘外側和下視丘的室旁核(paraventricular nucleus),在食慾的調節中扮演重要角色。當集中投射神經元受到活化時,可促進食慾,受瘦蛋白、胰島素和YY肽抑制;胃飢餓激素活化。
集中投射神經元包含阿黑皮素原(pro-opiomelanocortin,POMC)的產物、古柯鹼和安非他命調節轉錄(cocaine- and amphetamine-regulated transcript,CART)。集中投射神經元廣泛分布於腦內許多區域,包含下視丘全部的細胞核。這些細胞對於食慾的調控非常重要,當細胞受活化時會抑制進食。集中投射神經元受瘦蛋白和胰島素的循環濃度活化,且它們可直接促使肌肉活動;受NPY神經元抑制。
POMC神經元突出至內側視前核(medial preoptic nucleus),與人類性行為有關,不論男性或女性。POMC的表現受性類固醇類(gonadal steroids)調節,NPY會調節POMC產物及β-內啡肽。
集中投射神經元可產生體抑素,位於室周核(periventricular nucleus)的神經內分泌生長抑素神經元,可調控不同種類生長素的分泌。
Source: Wikipedia
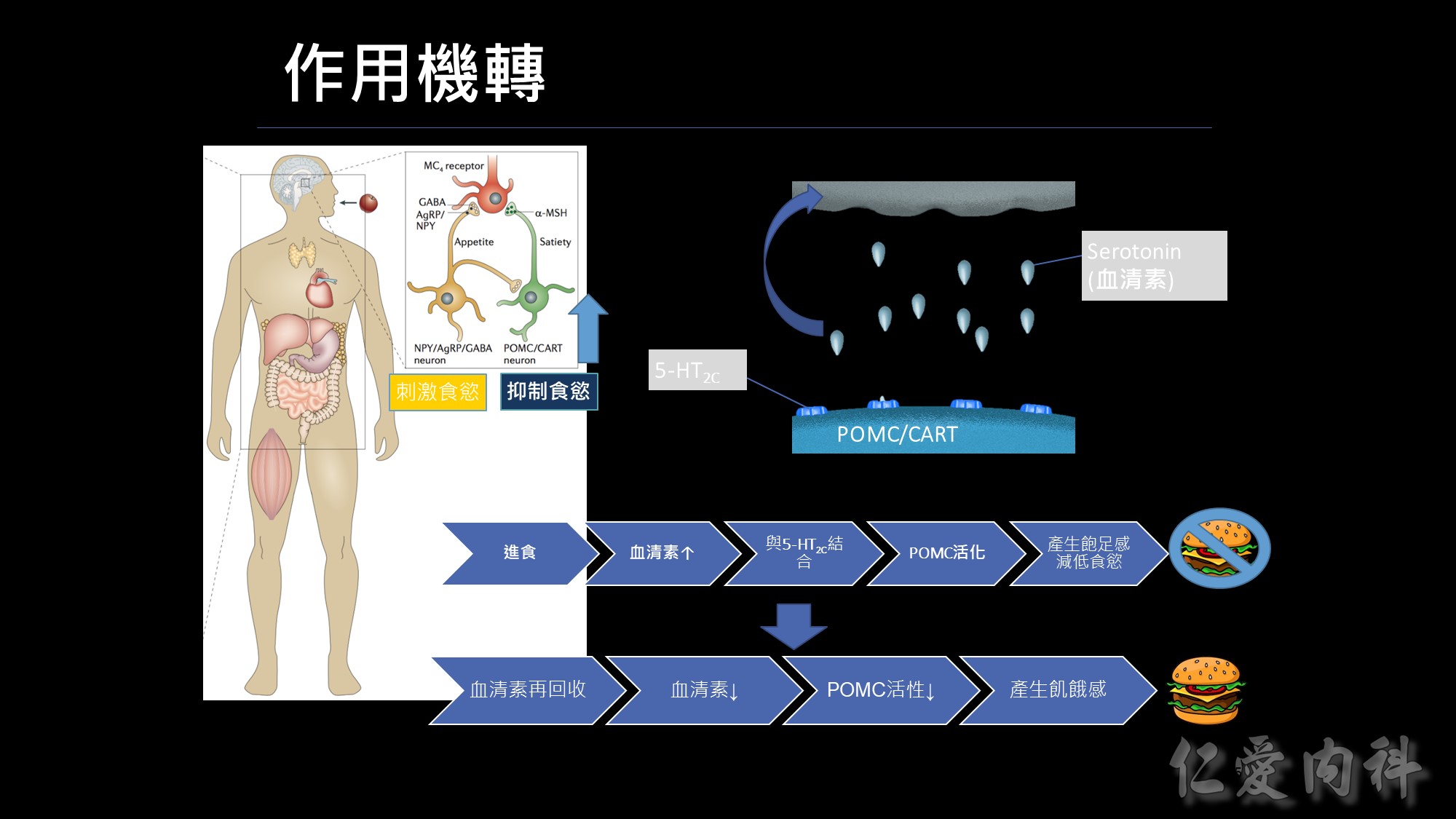
人體控制食慾的中樞是位於大腦的下視丘,分別為刺激食慾(agrp)及抑制食慾(POMC),我們的抑制中樞是位於POMC neuron上面,在這個POMC上面有個5-HT2c受體,這個表是一個人的身理機制,當我們進食後,體內血清素(serotonin,又稱5-羥色胺,簡稱為5-HT)會增加,這些血清素會跑到我們POMC neuron的受體5-HT2c作結合,而讓POMC 活化,進而產生飽足感,而不想再繼續進食。這樣的飽足感會維持一段時間;當然體內還是有平衡的機制會把血清素再回收,此時血清素濃度下降、POMC活性也下降,又再產生飢餓感。
下丘腦弓狀核(arcuate nucleus,ARC)是一個研究熱點。ARC內主要有兩個調節食慾神經環路的關鍵成分——AgRP/NPY神經元和POMC神經元,前者刺激食慾,而後者抑制食慾。
人體控制飢餓與飽足感的中樞是位於大腦,其中位於下視丘側部的位置,主要是發出訊號來刺激食慾,進而產生想吃東西的感覺調節食慾的關鍵就是-NPY與POMC神經元

前一代的減重藥品諾美婷是如何作用的呢?RD 的作用機轉是腎上腺素及血清素再回收抑制劑,把再回收的機制抑制掉,維持血清素的濃度、延長飽足感的時間,但這樣的作用機轉有一個問題~
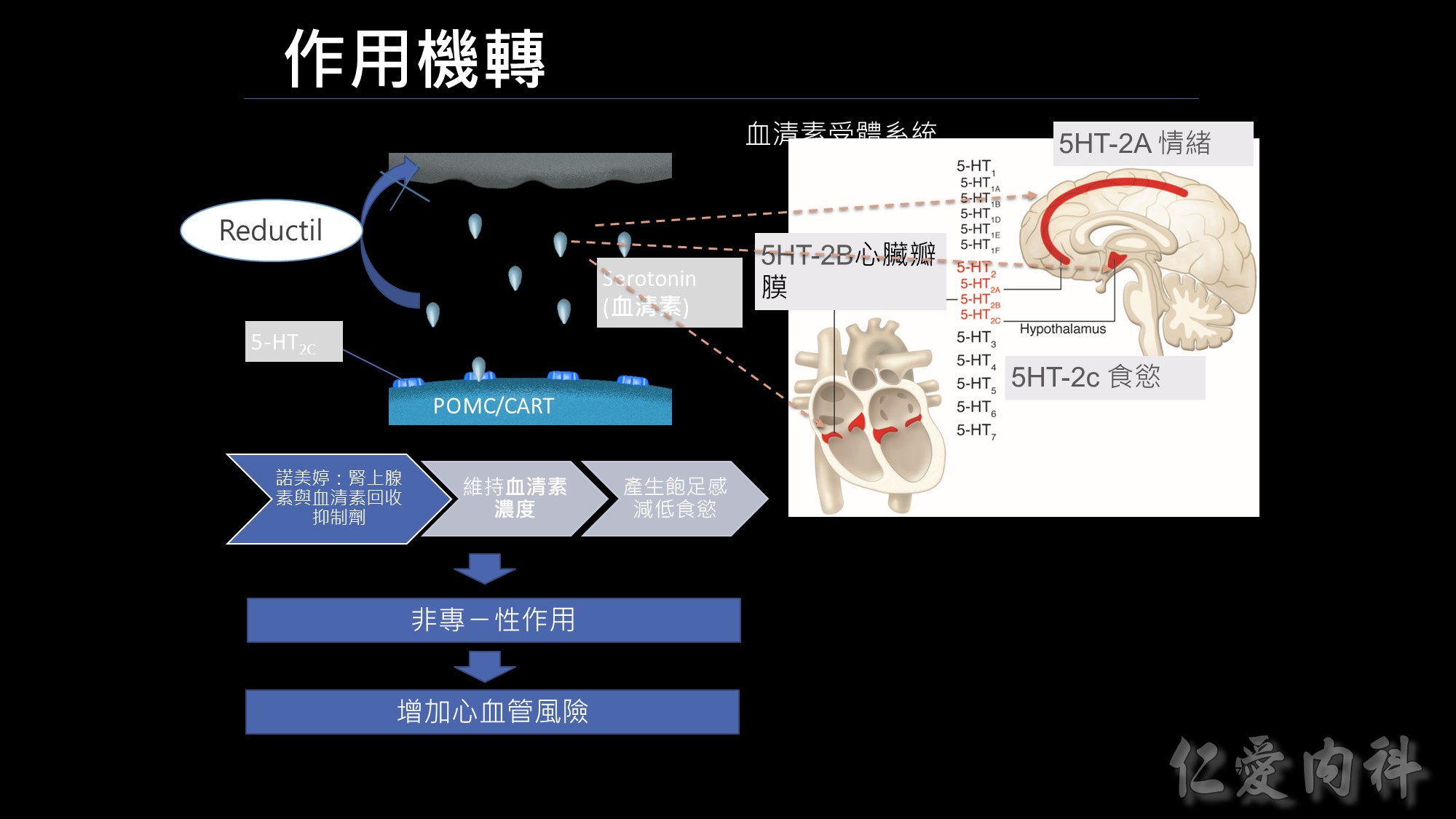
有什麼問題呢? 我們身體血清素的受體,不只在我們下視丘有,在我們大腦皮層也有大腦皮層是管理情緒,另外在我們的心臟瓣膜也有血清素結合的受體; 因為諾美婷不只作用在控管食慾的受體,非專一性的作用連帶影響到2A及2B,可能會增加情緒及心血管的風險,最後在2010年下市。
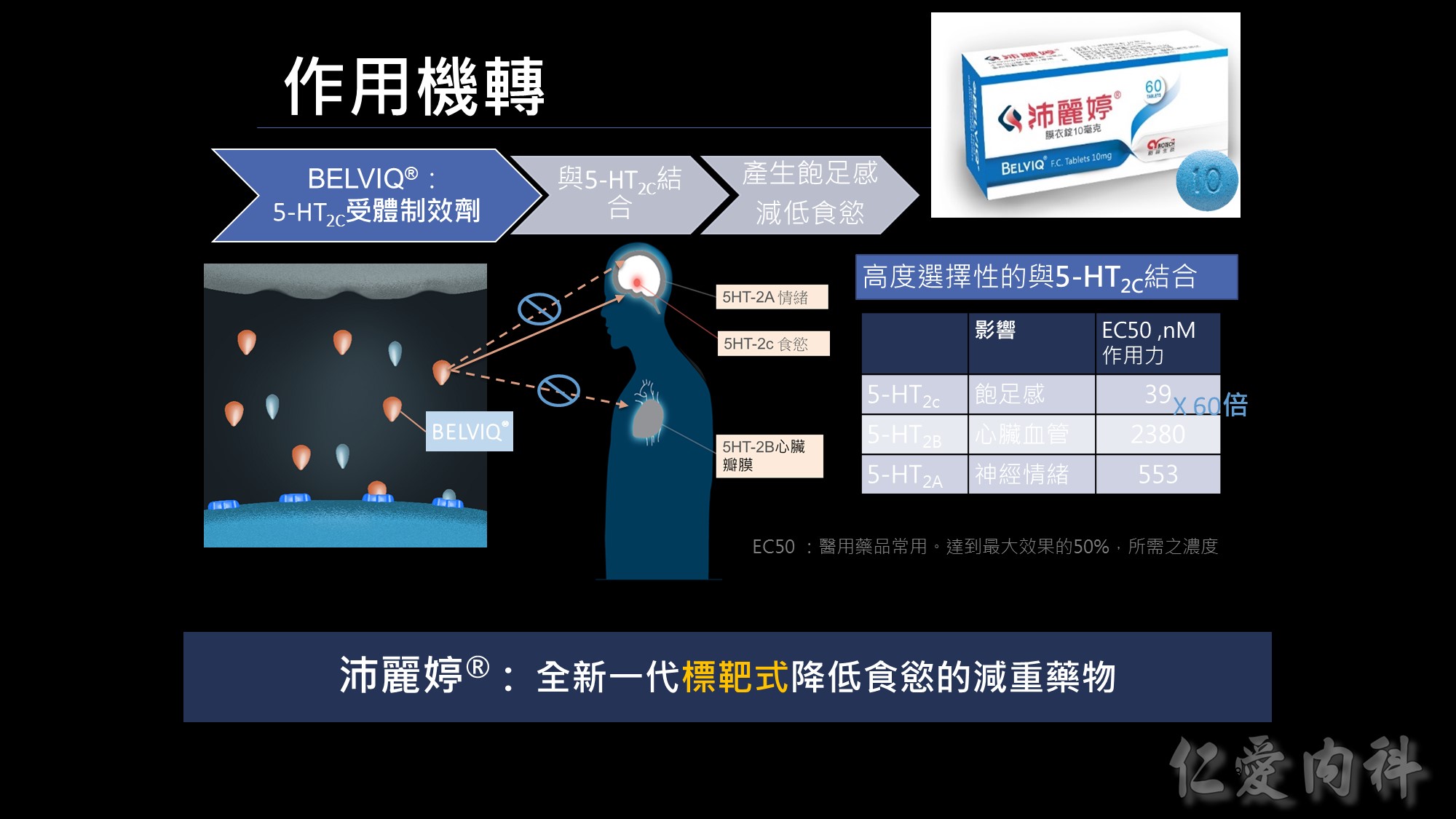
沛麗婷是一個全新的機轉; 它類似標靶藥的概念,不會影響情緒及心血管; 它能專一的選擇作用在掌控食慾的5-HT2c, 進而達成降低食慾的減重藥物。
若用藥品的作用力EC50來看,數字愈低親和力愈好,BELVIQ的作用力是心血管的作用力60倍;<我們從後續的臨床實驗結果也證實不會影響CV &情緒>所以我們的BELVIQ是更安全、新一代抑制食慾的減重新藥。
會不會影響到2A、2B?
不過雖然說Lorcaserin是選擇性作用於5-HT2C 受體,但其結合到不同受體的親合力呈劑量依賴性,也就是說當劑量不超過每日20毫克時,Lorcaserin呈現選擇性作用於5-HT2C 受體,而不作用於5-HT2A及5-HT2B受體,故副作用低。但若服用劑量超過每日20毫克時,Lorcaserin就有可能作用在5-HT2A及5-HT2B受體,而引起幻覺、欣快感(5-HT2A),甚至造成心血管副作用(5-HT2B)。

口服1.5-2小時可達血中最高濃度
連續服藥3天後可達血中穩定濃度
5個半衰期(55小時/2.5天)可完全代謝完
肝臟代謝、尿液排出
適用之身體質量指數為:
(1) BMI ≧ 30 kg/m2 或 (2) BMI ≧ 27 kg/m2 且有一項與體重相關之疾病 (例如: 高血壓、血脂異常或第二型糖尿病)。
- BELVIQ 併用其他減重藥物(處方藥、非處方藥及中草藥)之安全性和藥效性尚未確立
- BELVIQ 對於心血管系統之影響(致病率及致死率)尚未確立。
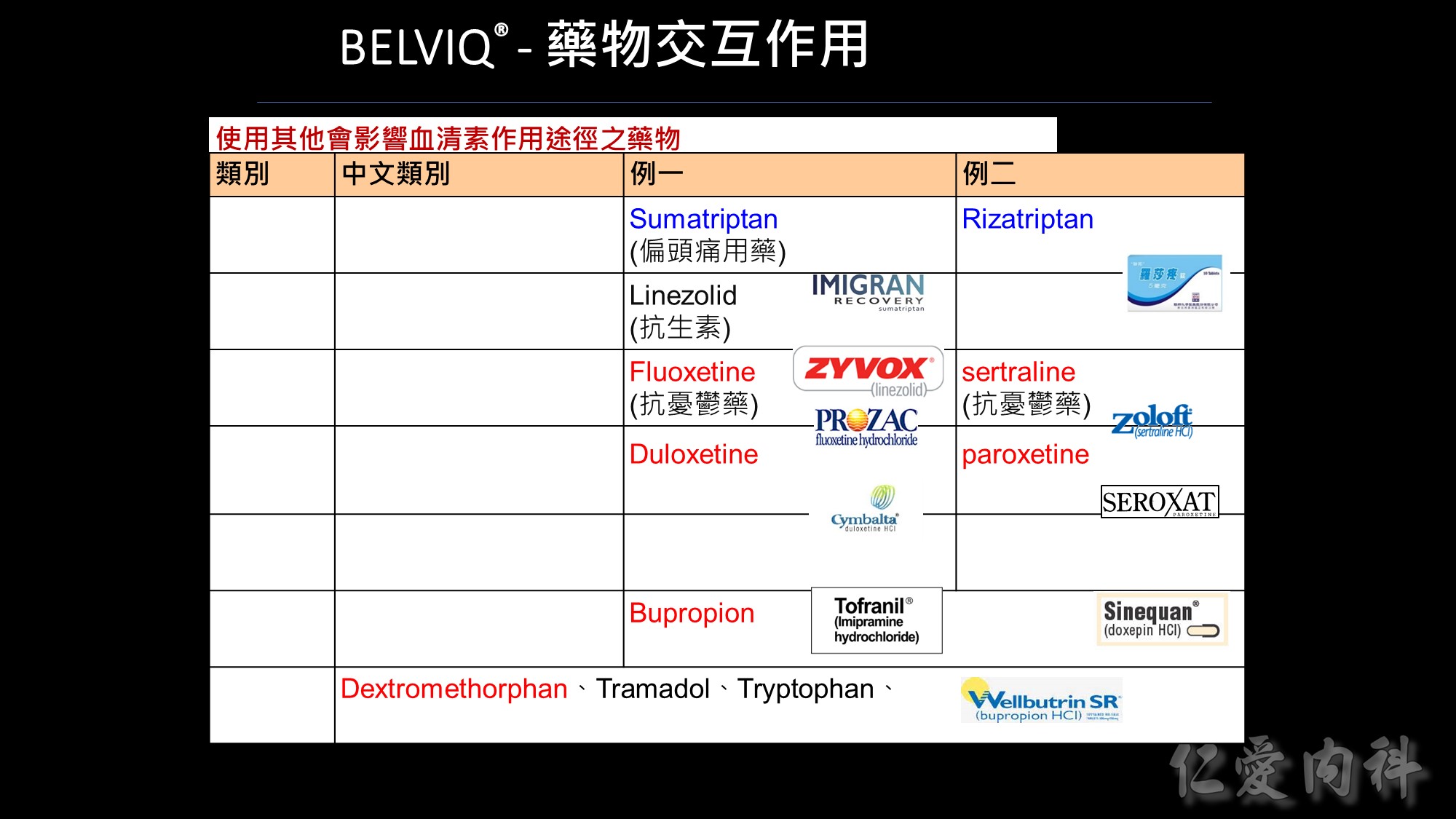
使用其他會影響血清素作用途徑之藥物
基於 BELVIQ 的作用機轉和理論上會發生血清素症候群的潛在可能,應特別小心使用其他會影響血清素作用途徑之藥物,包括但不限於:triptans、單胺氧化酶抑製劑(包括 linezolid, 一種抗生素,可逆的非選擇性單胺氧化酶抑製劑)、選擇性血清素再回收抑製劑(SSRIs)、選擇性血清素正腎上腺素再回收抑製劑(SNRIs)、dextromethorphan、三環抗憂鬱藥、bupropion、鋰劑、tramadol、tryptophan 和聖約翰草。
經由CYP 2D6 進行代謝之藥物
當藥物在體內主要是經由CYP 2D6 進行代謝時,該藥物在併用BELVIQ 時需特別小心,因為BELVIQ 可以提高這些藥物在體內的暴露量。


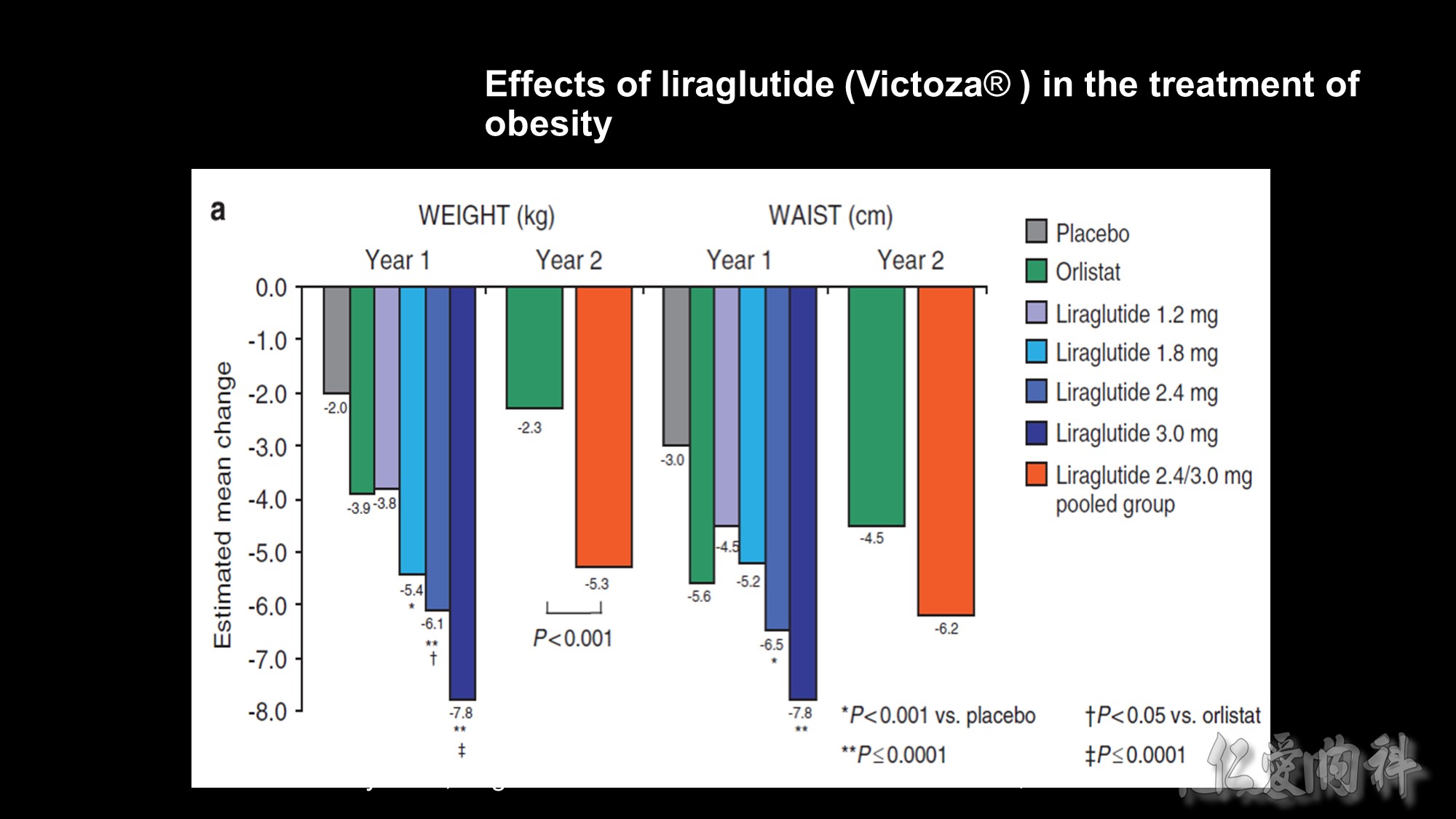
Background: The frequency of obesity has risen dramatically in recent years but only few safe and effective drugs are currently available. We assessed the eff ect of liraglutide on bodyweight and tolerability in obese individuals without type 2 diabetes.
Methods: We did a double-blind, placebo-controlled 20-week trial, with open-label orlistat comparator in 19 sites in Europe. 564 individuals (18–65 years of age, body-mass index 30–40 kg/m2) were randomly assigned, with a telephone or web-based system, to one of four liraglutide doses (1·2 mg, 1·8 mg, 2·4 mg, or 3·0 mg, n=90–95) or to placebo (n=98) administered once a day subcutaneously, or orlistat (120 mg, n=95) three times a day orally. All individuals had a 500 kcal per day energy-defi cit diet and increased their physical activity throughout the trial, including the 2-week run-in. Weight change analysed by intention to treat was the primary endpoint. An 84-week open-label extension followed. This study is registered with ClinicalTrials.gov, number NCT00422058.
Findings: Participants on liraglutide lost signifi cantly more weight than did those on placebo (p=0·003 for liraglutide 1·2 mg and p<0·0001 for liraglutide 1·8–3·0 mg) and orlistat (p=0·003 for liraglutide 2·4 mg and p<0·0001 for liraglutide 3·0 mg). Mean weight loss with liraglutide 1·2–3·0 mg was 4·8 kg, 5·5 kg, 6·3 kg, and 7·2 kg compared with 2·8 kg with placebo and 4·1 kg with orlistat, and was 2·1 kg (95% CI 0·6–3·6) to 4·4 kg (2·9–6·0) greater than that with placebo. More individuals (76%, n=70) lost more than 5% weight with liraglutide 3·0 mg that with placebo (30%, n=29) or orlistat (44%, n=42). Liraglutide reduced blood pressure at all doses, and reduced the prevalence of prediabetes (84–96% reduction) with 1·8–3·0 mg per day. Nausea and vomiting occurred more often in individuals on liraglutide than in those on placebo, but adverse events were mainly transient and rarely led to discontinuation of treatment.
Interpretation: Liraglutide treatment over 20 weeks is well tolerated, induces weight loss, improves certain obesity-related risk factors, and reduces prediabetes.