作者/講者: 張哲誠 醫師
筆記與整理: 陳羿方 醫師
校稿: Ian YC Chen, MD
上次校閱: 2018/09/22
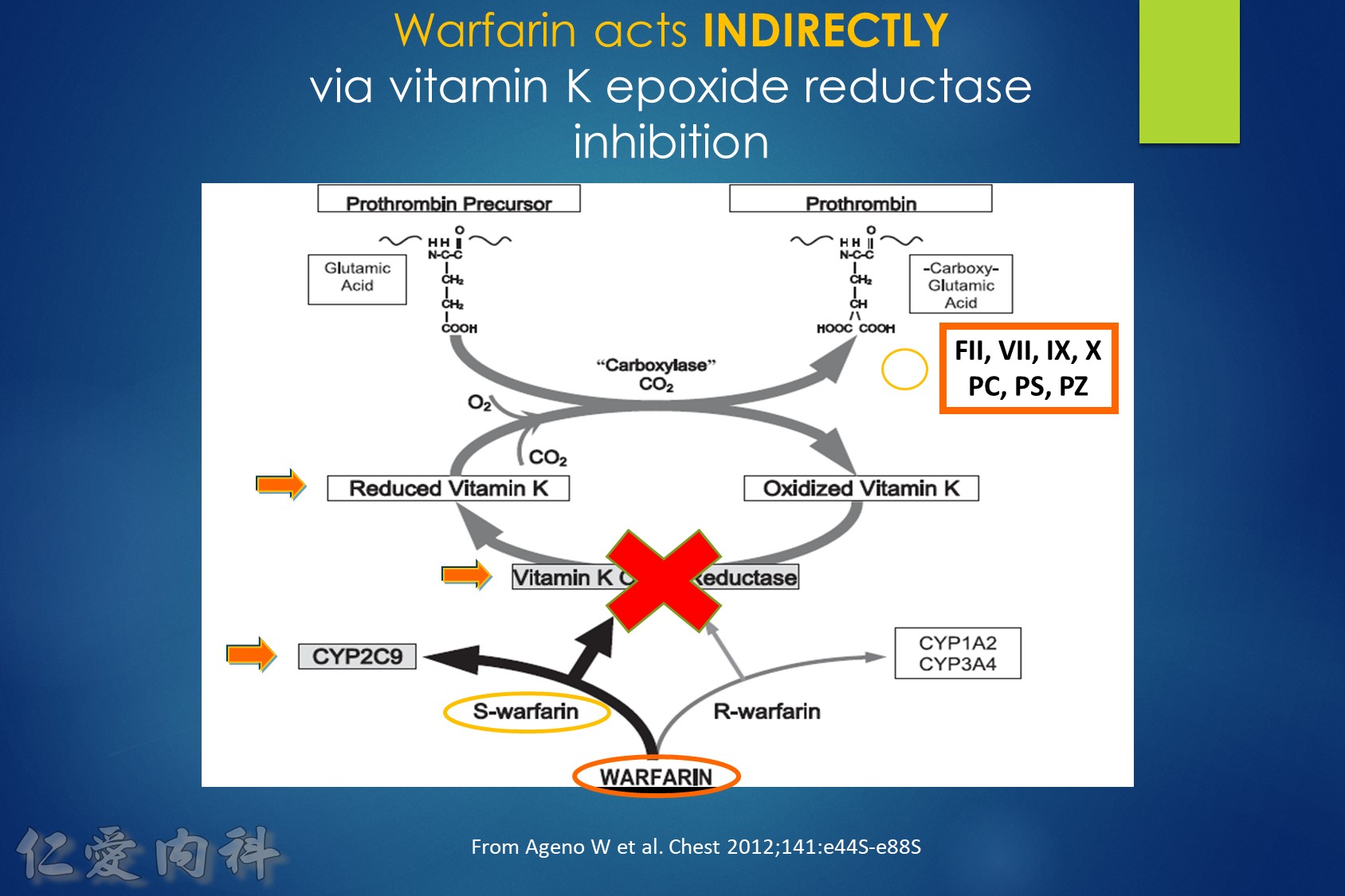
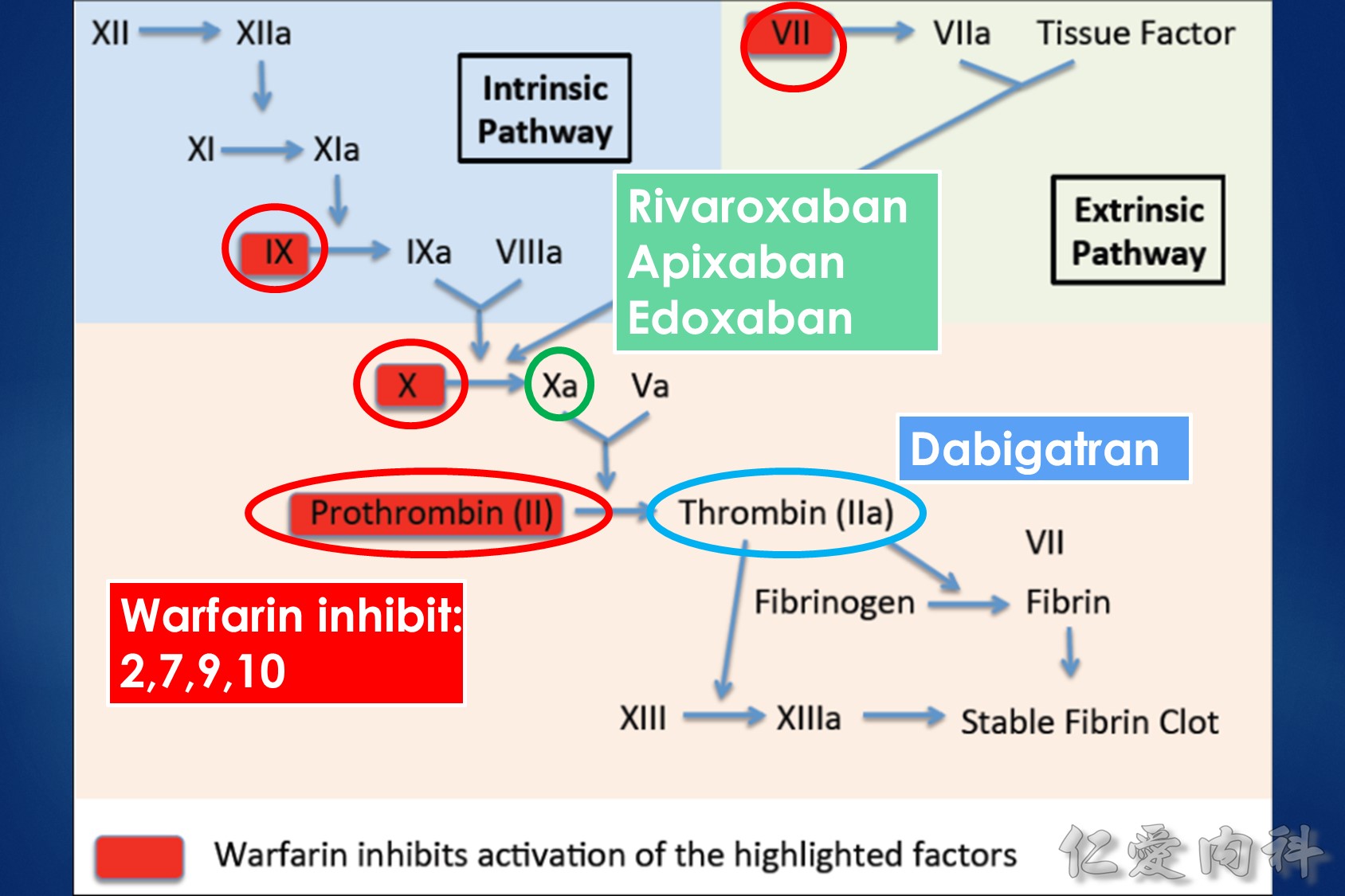
Fig1:
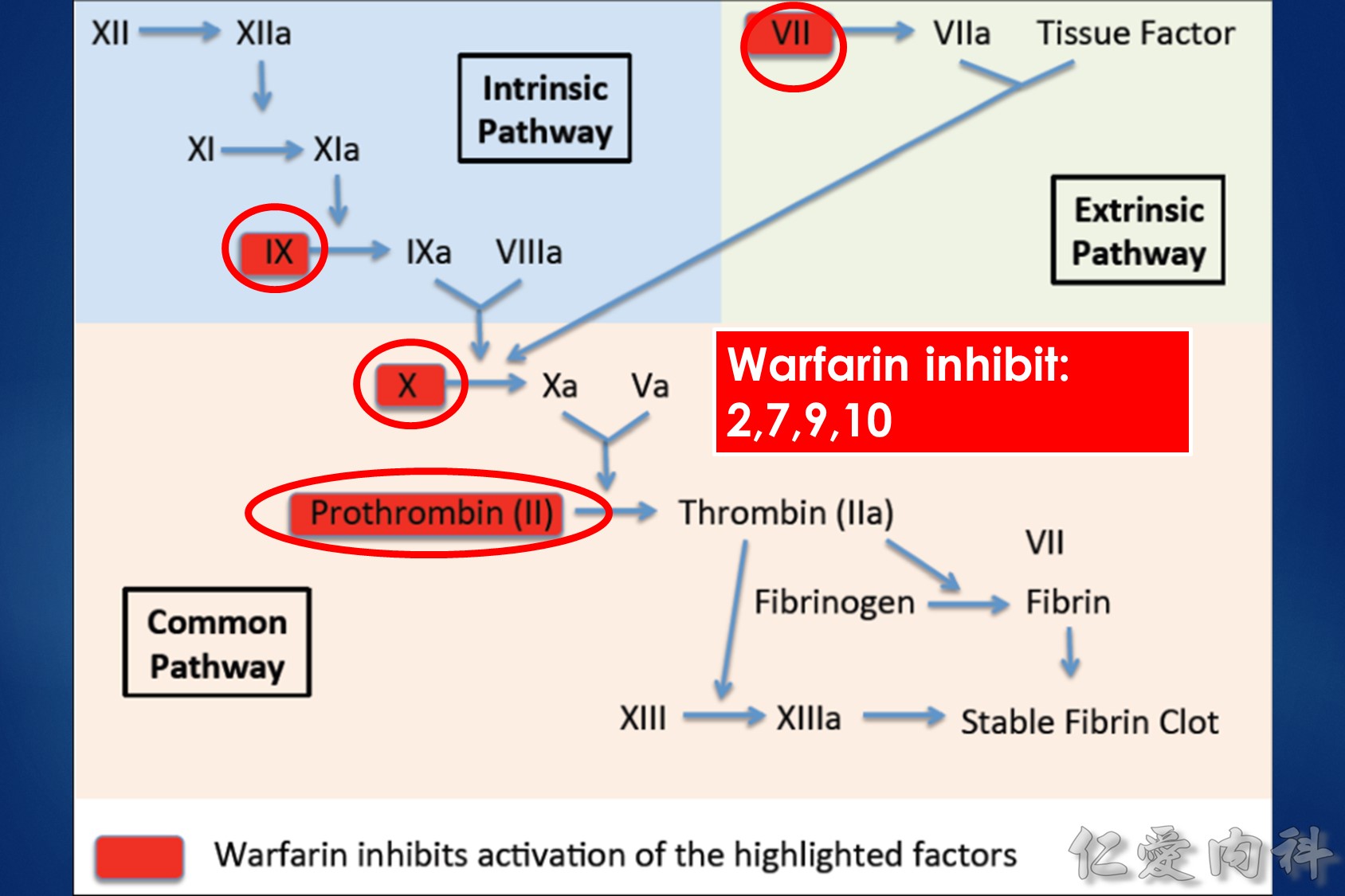
The interrelationships between vitamin K, coagulation factors, and warfarin. Prothrombin, representative of the vitamin K-dependent coagulation factors (II, VII, IX, and X), requires reduced vitamin K to become fully functional. Vitamin K is oxidized and then recycled. S-warfarin’s pharmacologic effect is mediated by interfering with the vitamin K oxide reductase enzyme and the recycling of vitamin K. S-warfarin is metabolized by P450 enzyme CYP2C9.
Mentions:
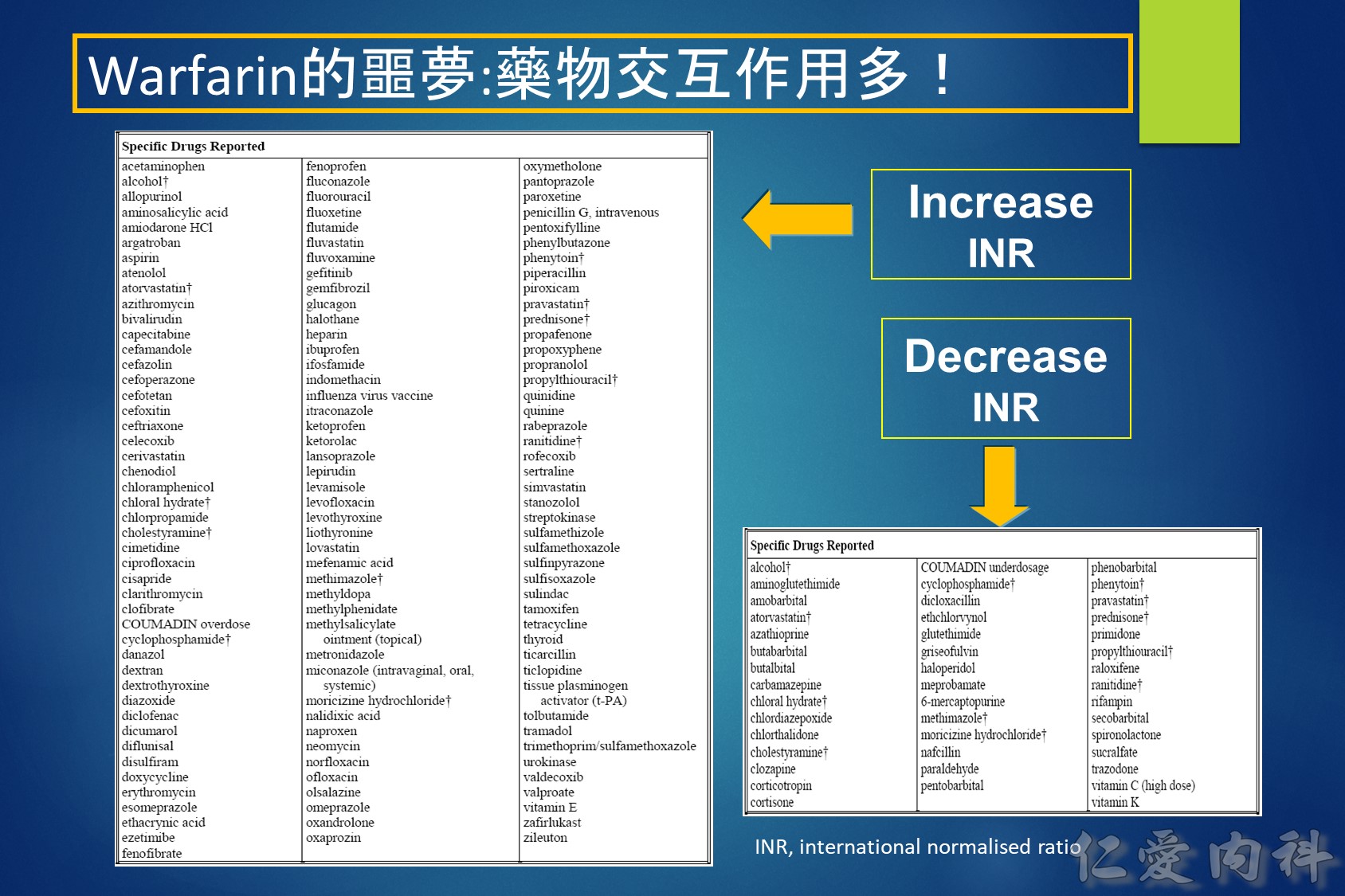
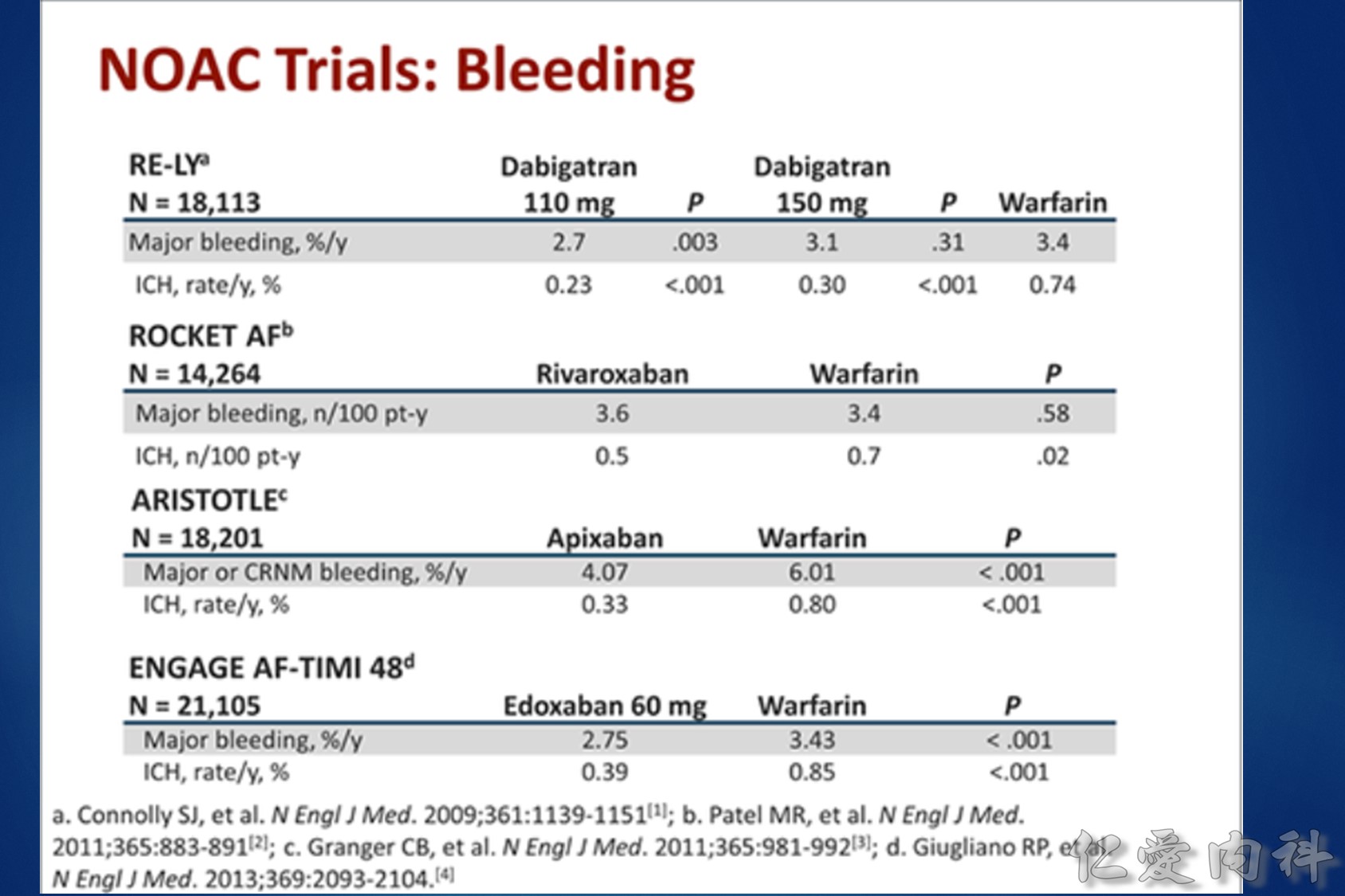
Warfarin is a stereo isomer of two enantiomers, the S enantiomer having the principal biologic effect of interfering with vitamin K metabolism (Fig. 1).6 S-warfarin is metabolized by the P450 cytochrome oxidase, 2C9. Two relatively common single nucleotide polymorphisms are responsible for altering the metabolic function of CYP2C9 leading to impaired drug metabolism, accumulation of warfarin, and further elevation of the INR. Warfarin’s major target is the vitamin K oxide reductase complex 1 (VKORC1). Several polymorphisms in combination lead to haplotypes with either enhanced or reduced sensitivity to warfarin. In addition to genetic factors that affect warfarin’s pharmacokinetic and pharmacodynamic behavior, warfarin effect is also influenced by age, gender, body surface area, dietary vitamin K content, liver function, and multiple drug interactions. Thus, pharmacogenetic-based dosing by itself is not a solution to improved therapeutic effectiveness, but must be considered along with these other factors. Complex dosing equations, some of which are freely available on the web, incorporate many of these factors.
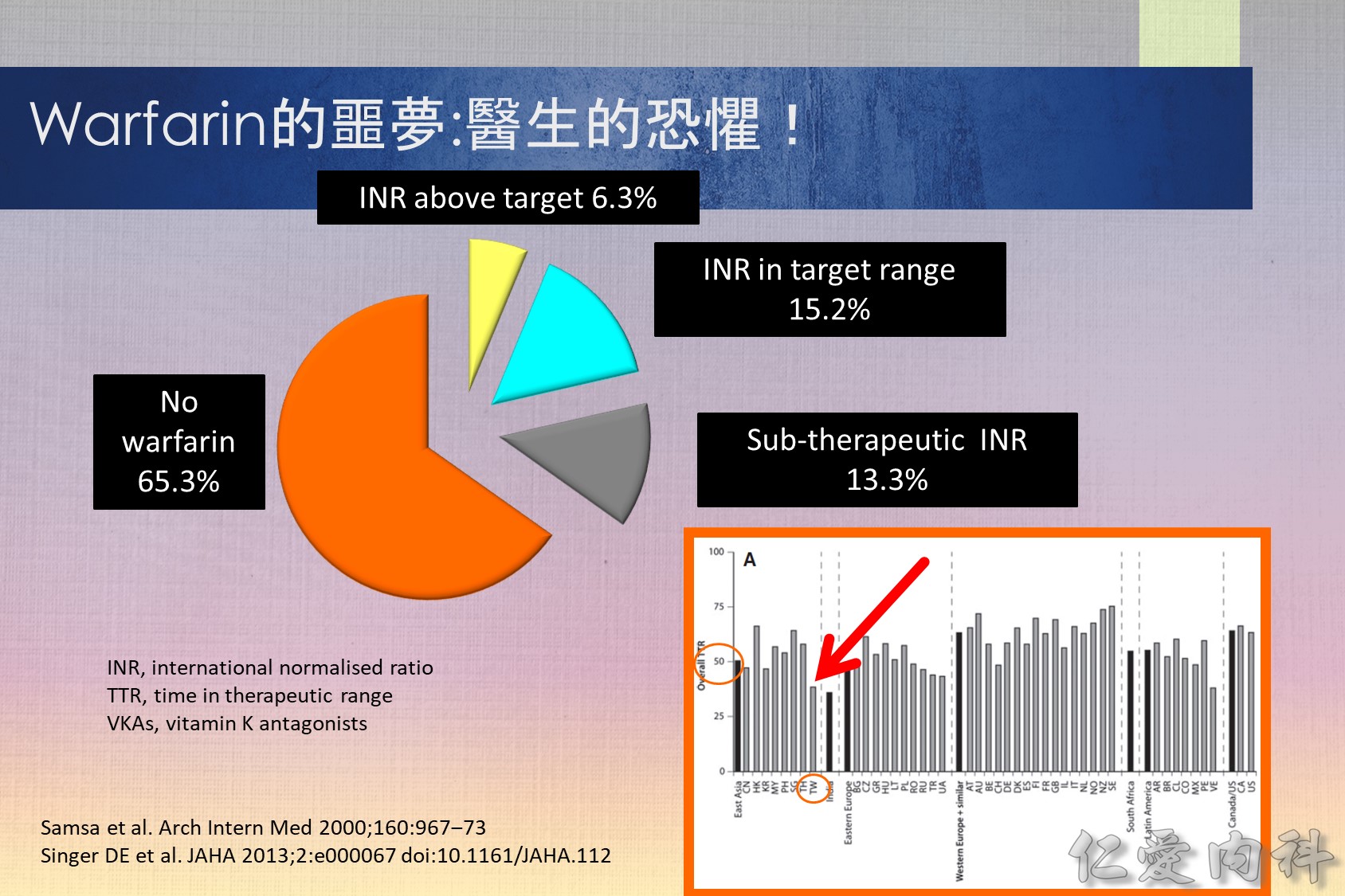
Vitamin K antagonists (VKAs) i.e. warfarin, are the most commonly used oral anti-coagulants. However, some studies have repored that 56–85% of eligible patients do not actually receive treatment.1,2 Furthermore, the lowest utilization levels are in the very elderly, those at the highest risk of stroke.3 It appears that some of the reasons for this underuage of VKAs are4: (1) comorbidities of patients; (2) overcautious prescribing by physicians; (3) Non-compliance by patients (due to complex dosing regimens and frequent monitoring); (4) patient preference. Even in patients who receive treatment with warfarin, it appears that for the majority it is sub-optimal with patients‘ INR being out of the target range.2
References
- Bungard et al. Arch Intern Med 2000; 160:41–46
- Samsa et al. Arch Intern Med 2000;160:967–973
- Go et al. Ann Intern Med 1999; 131: 927–934
- Ansell et al. Chest 2004;126 (3 Suppl):204S–233S
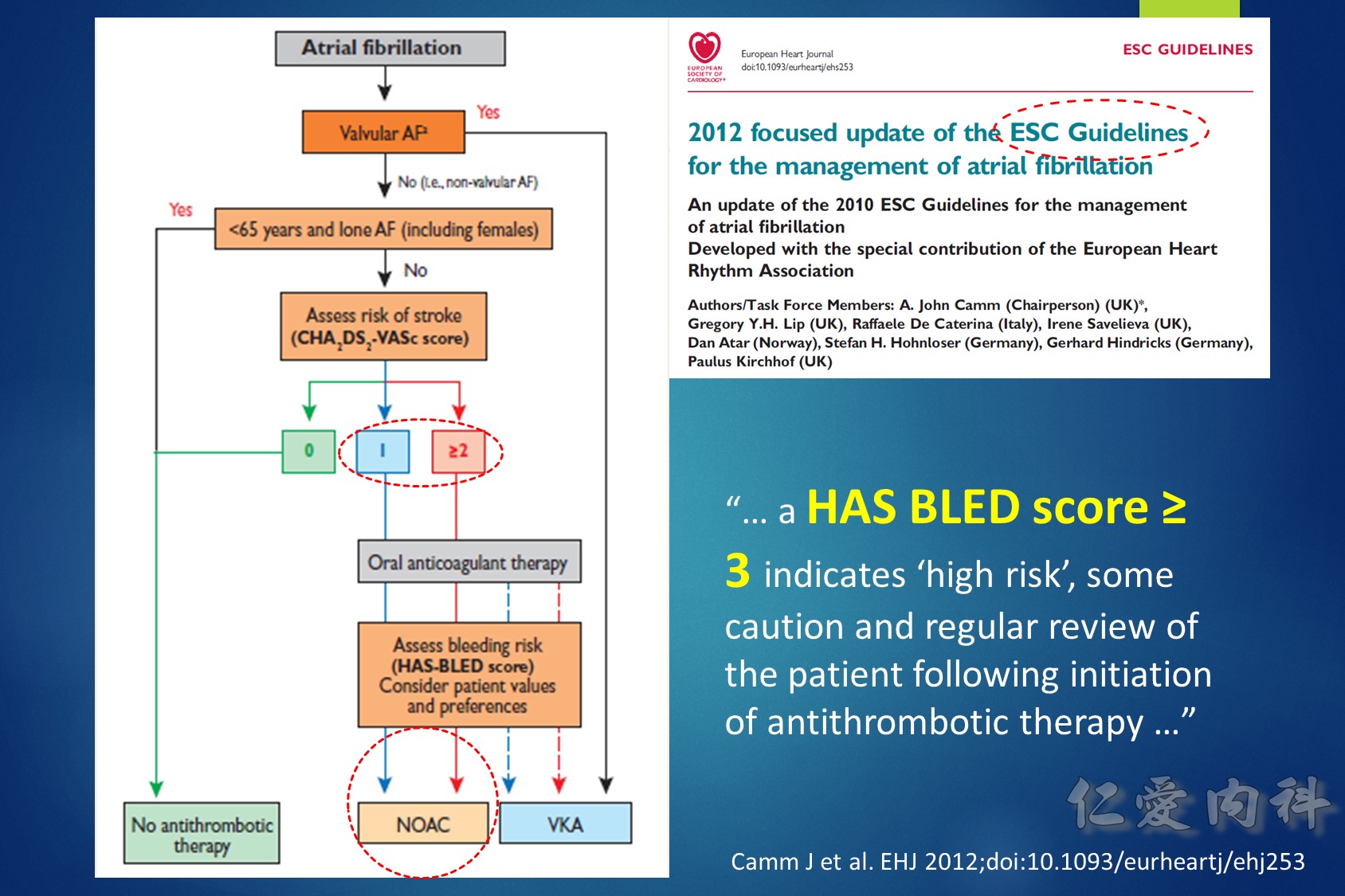
目前大都採用 TTR (time in therapeutic range) 如 TTR 65% 代表患者在治療期間平均有 65% 的時 間維持在治療範圍之內,許多大型試驗利用來 評估口服抗凝血劑的治療成果和品質,TTR 愈高,中風、出血、死亡的發生率愈低 。在品 質理想的大型試驗 TTR 應維持在 66% 左右,但在歐洲心臟調查 (Euro Heart Survey) 有超過50% 達不到理想的抗凝血治療,真實的臨床實務只有 44% 的水準,同時在不同國家, 甚至同一國家的不同地區存在很大的差異。(內科學誌 2012:23:77-97)
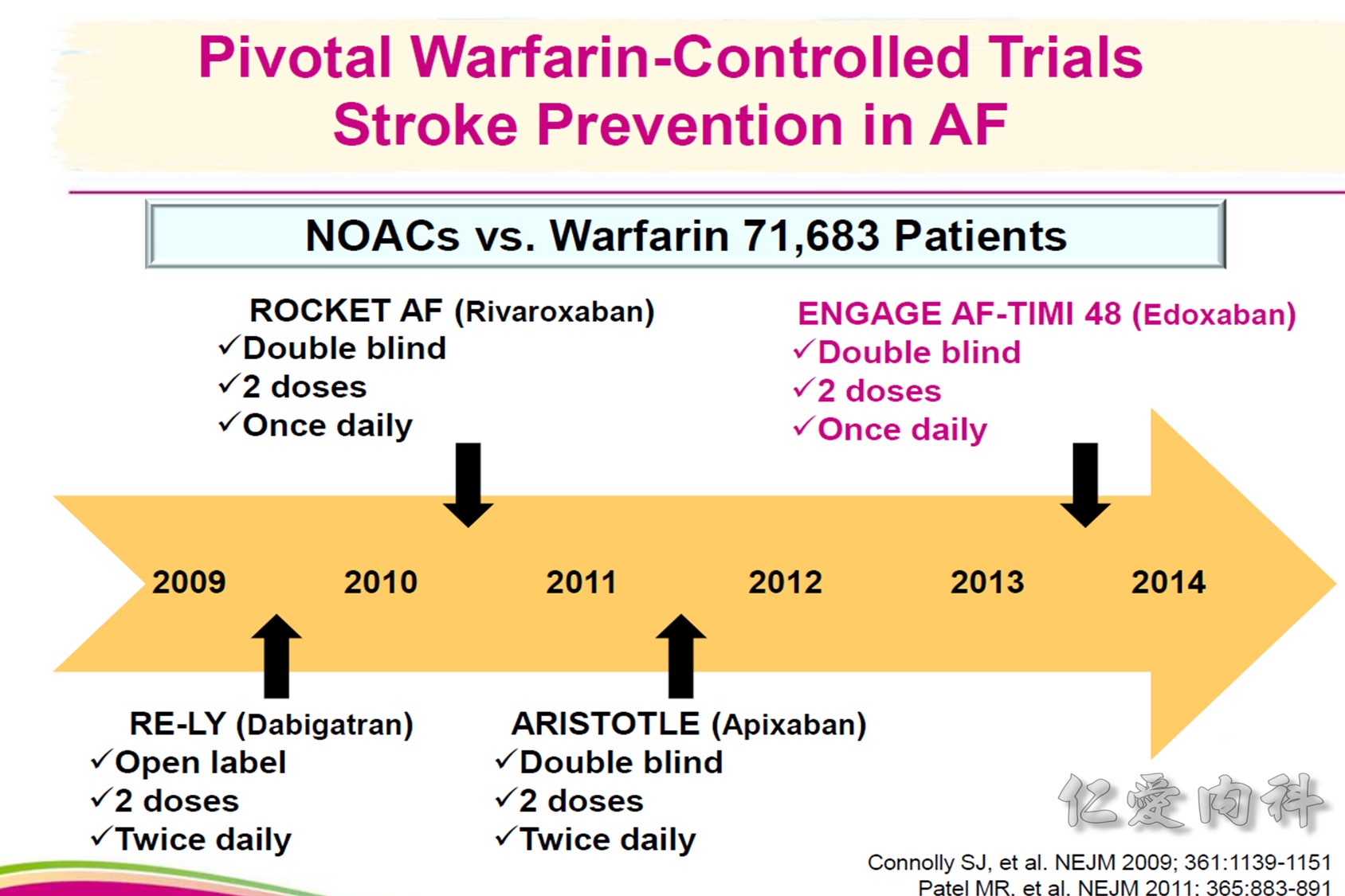
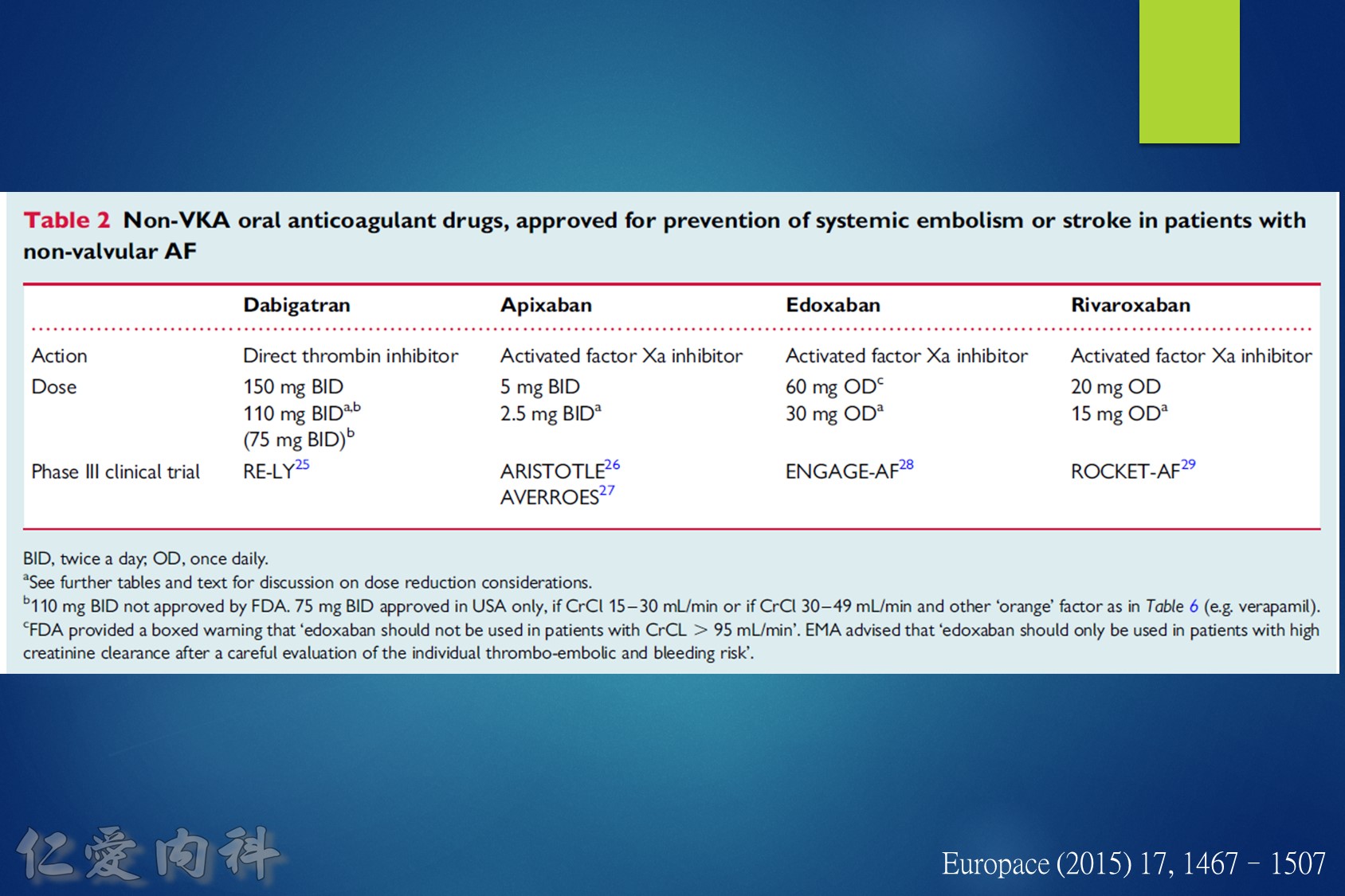
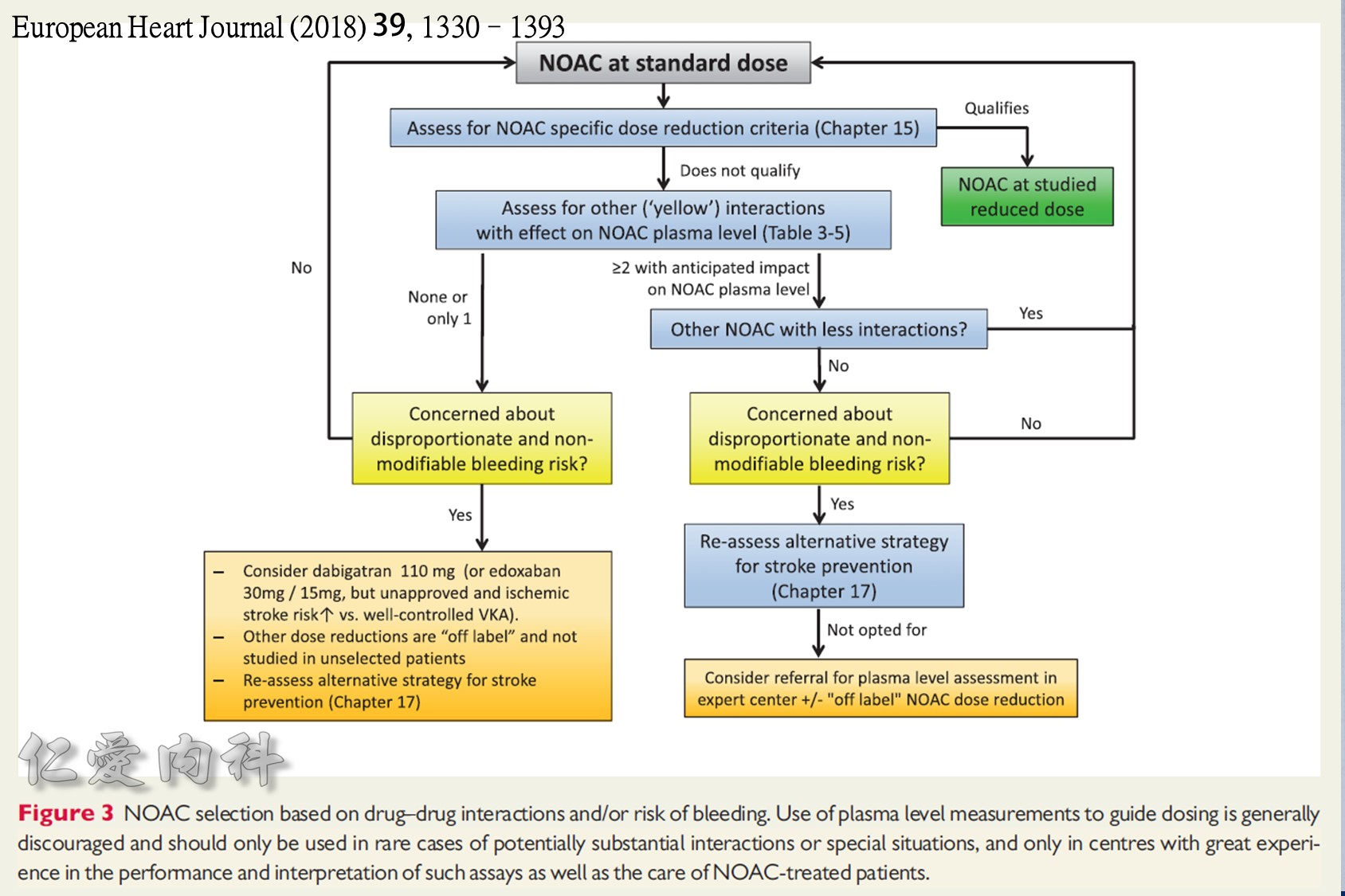
2018 EHRA Practical Guide to NOAC Use in AF
Use of NOACs is contraindicated for AF patients with mechanical prosthetic valves or moderate-severe mitral stenosis (usually of rheumatic origin). Although there are limited data for patients with bioprosthetic valves, mitral valve repair, or transcatheter aortic valve replacement, use is acceptable.
The following are key points to remember from this practical guide on the use of nonvitamin K antagonist oral anticoagulants (NOACs) in patients with atrial fibrillation (AF) from the European Heart Rhythm Association (EHRA):
1.Use of NOACs is contraindicated for AF patients with mechanical prosthetic valves or moderate-severe mitral stenosis (usually of rheumatic origin). Although there are limited data for patients with bioprosthetic valves, mitral valve repair, or transcatheter aortic valve replacement, use is acceptable.
2.Structured follow-up is recommended for patients on chronic NOAC use. This includes documentation of anticoagulation indication, checking baseline laboratory studies (hemoglobin, renal and liver function, coagulation panel), providing education, and coordinating follow-up with at least once-yearly laboratory studies. Repeat laboratory testing should be performed more frequently for patients with baseline renal dysfunction or the elderly.
3.It is important to calculate renal function using the Cockcroft-Gault equation. This is used to appropriately dose NOACs. Use of NOACs is not recommended for patients with creatinine clearance <15-30 ml/min or on dialysis. Note: In the United States, apixaban is dosed based on serum creatinine level, weight, and age (this differs from European dosing). Also, both apixaban and rivaroxaban are Food and Drug Administration (FDA) approved for use with dialysis.
4.It is also important to check baseline liver function before starting NOACs. NOACs are contraindicated for patients with Child-Pugh category C hepatic insufficiency. Rivaroxaban is also contraindicated in Child-Pugh category B hepatic insufficiency.
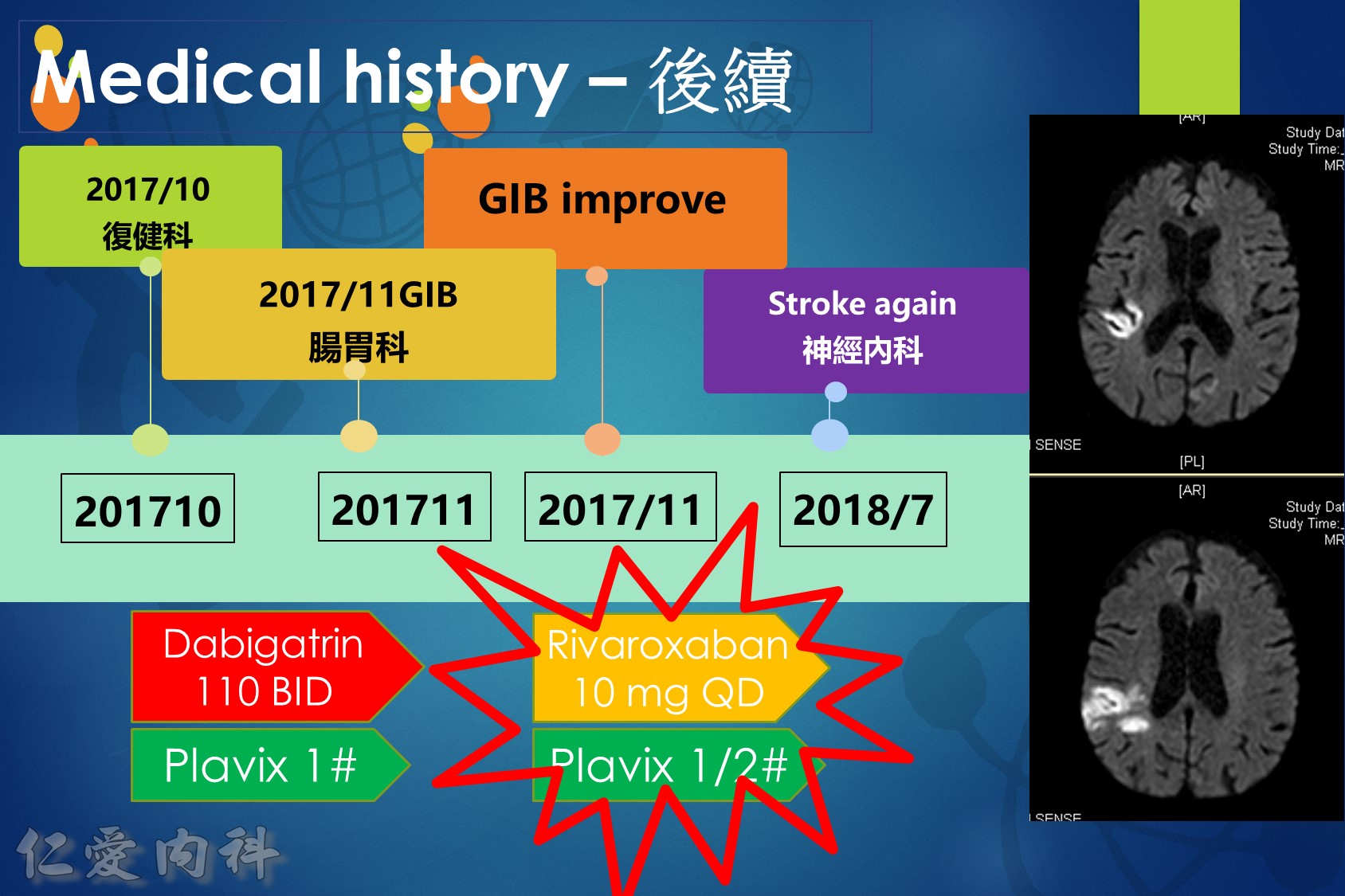
5.When switching from warfarin to a NOAC, the NOAC should be started when the international normalized ratio (INR) is <2.5. When switching from a NOAC to warfarin, warfarin should be started and the NOAC continued until the INR is ≥2. Repeat the INR 1-3 days after stopping NOAC to ensure INR remains therapeutic.
6.Although NOACs have fewer drug-drug interactions than warfarin, there are still important drug-drug interactions to monitor. These include the P-glycoprotein and CYP3A4 interacting medicines. Important examples include avoiding concurrent use of dronedarone, rifampin, many HIV protease inhibitors, itraconazole, ketoconazole, voriconazole, St. John’s wort, and dexamethasone.
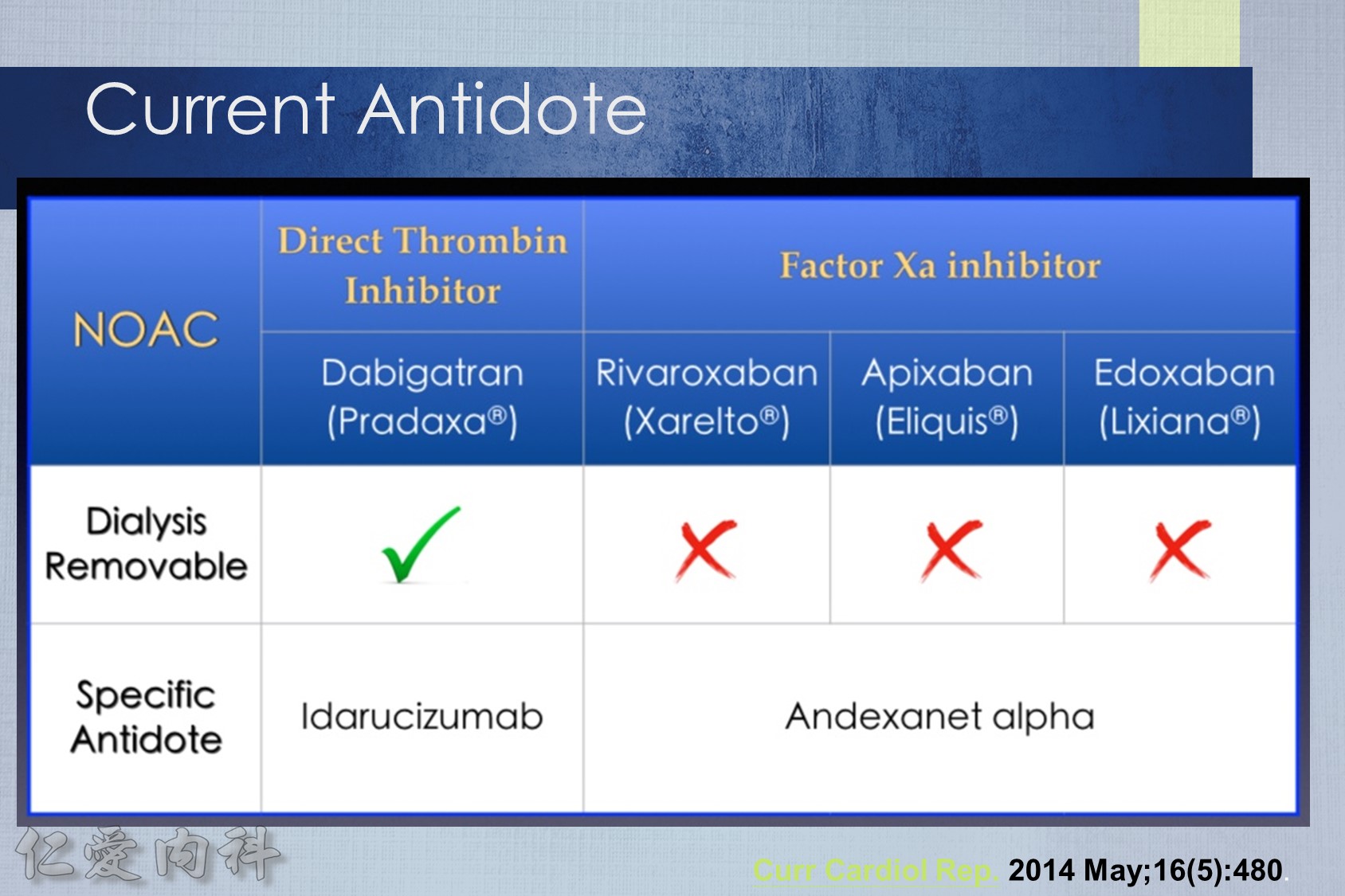
7.In the case of a nonlife-threatening major bleeding event, plasma levels of NOACs should normalize within 12-24 hours for patients with normal renal function. It may take longer for patients with renal insufficiency, particularly for dabigatran.
8.In the case of a life-threatening major bleeding event, patients on dabigatran can be given idarucizumab 5 mg IV in two doses no more than 15 minutes apart. Patients taking factor Xa inhibitors should be given prothrombin complex concentrate 50 U/kg. All patients should receive supportive measures, including mechanical compression and endoscopic or surgical hemostasis (if applicable).
9.Following a major gastrointestinal bleeding event, NOACs should be restarted as early as feasible (usually 4-7 days) if the risk of stroke persists and outweighs the risk of recurrent bleeding.
10.Most patients taking NOACs can safely undergo surgical procedures with a 24- to 48-hour pre-procedure hold. Longer hold times may be necessary for patients taking dabigatran who have chronic kidney disease. No bridging heparin is needed for NOAC-treated patients. Resume full-dose NOAC within 72 hours post-procedure, once the bleeding risk is appropriate.
11.For patients taking NOAC who present with an acute coronary syndrome, primary percutaneous coronary intervention (PCI) can be performed (preferably using a radial approach) emergently for ST-segment elevation myocardial infarction (STEMI) patients or delayed for 24-48 hours in stable NSTEMI patients. Consider a proton pump inhibitor for patients taking combined NOAC with antiplatelet medications.
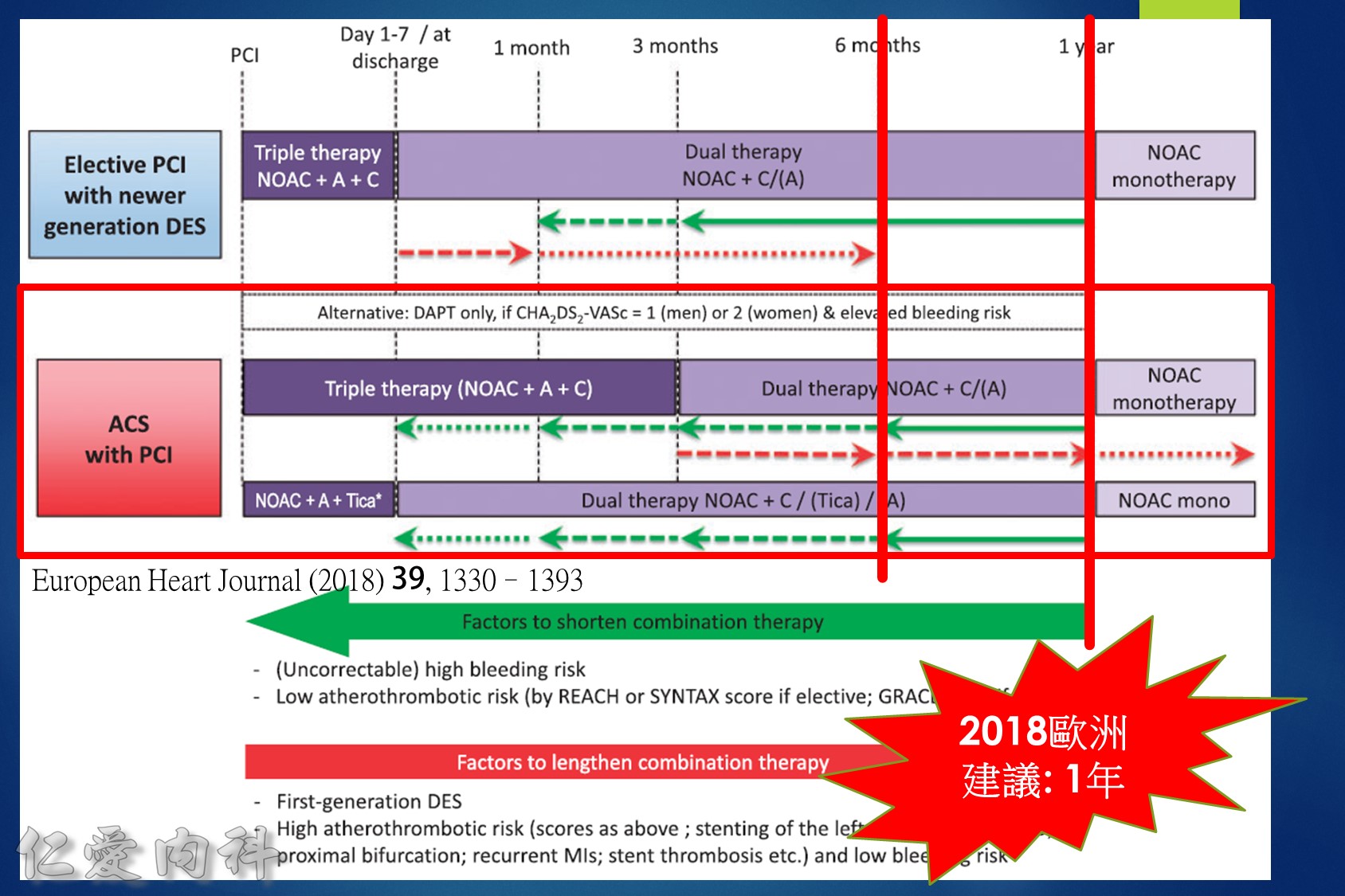
12.For patients taking NOAC with single or dual antiplatelet therapy, shorter courses of antiplatelets are recommended. Patients with elective PCI may benefit from dual therapy (NOAC plus clopidogrel from discharge through 1 year). Patients with acute coronary syndromes who undergo PCI should receive triple therapy for up to 3 months, then switch to dual therapy (NOAC plus clopidogrel) until 1 year. After 1 year, all patients should continue on NOAC monotherapy.
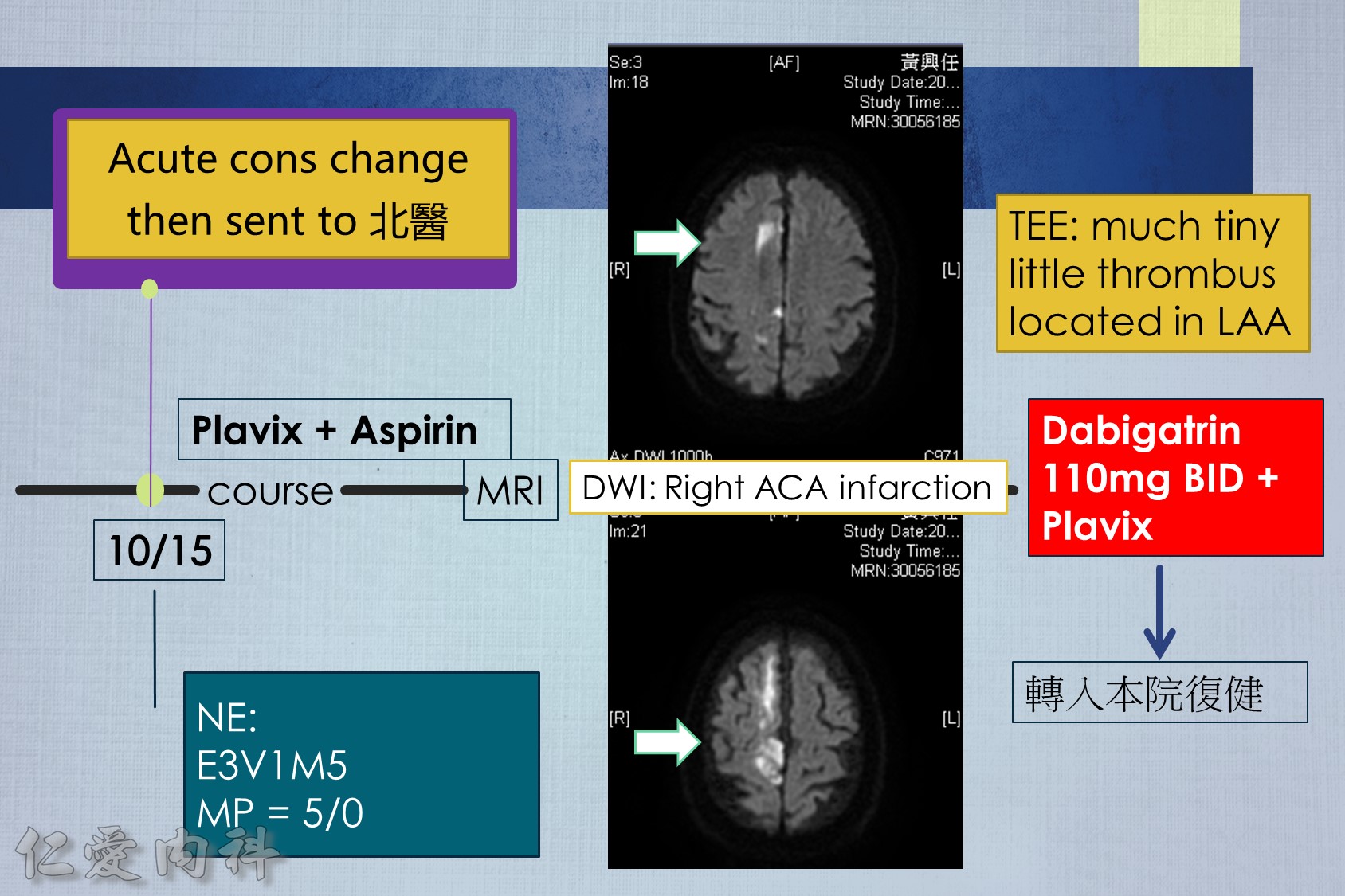
13.For patients taking NOAC who present with an acute ischemic stroke, proceed with thrombolysis if the NOAC plasma level is below the lower limit of detection or if the last intake was >48 hours prior and renal function is normal. Otherwise consider thrombolysis in select patients after NOAC reversal or use of endovascular therapy.
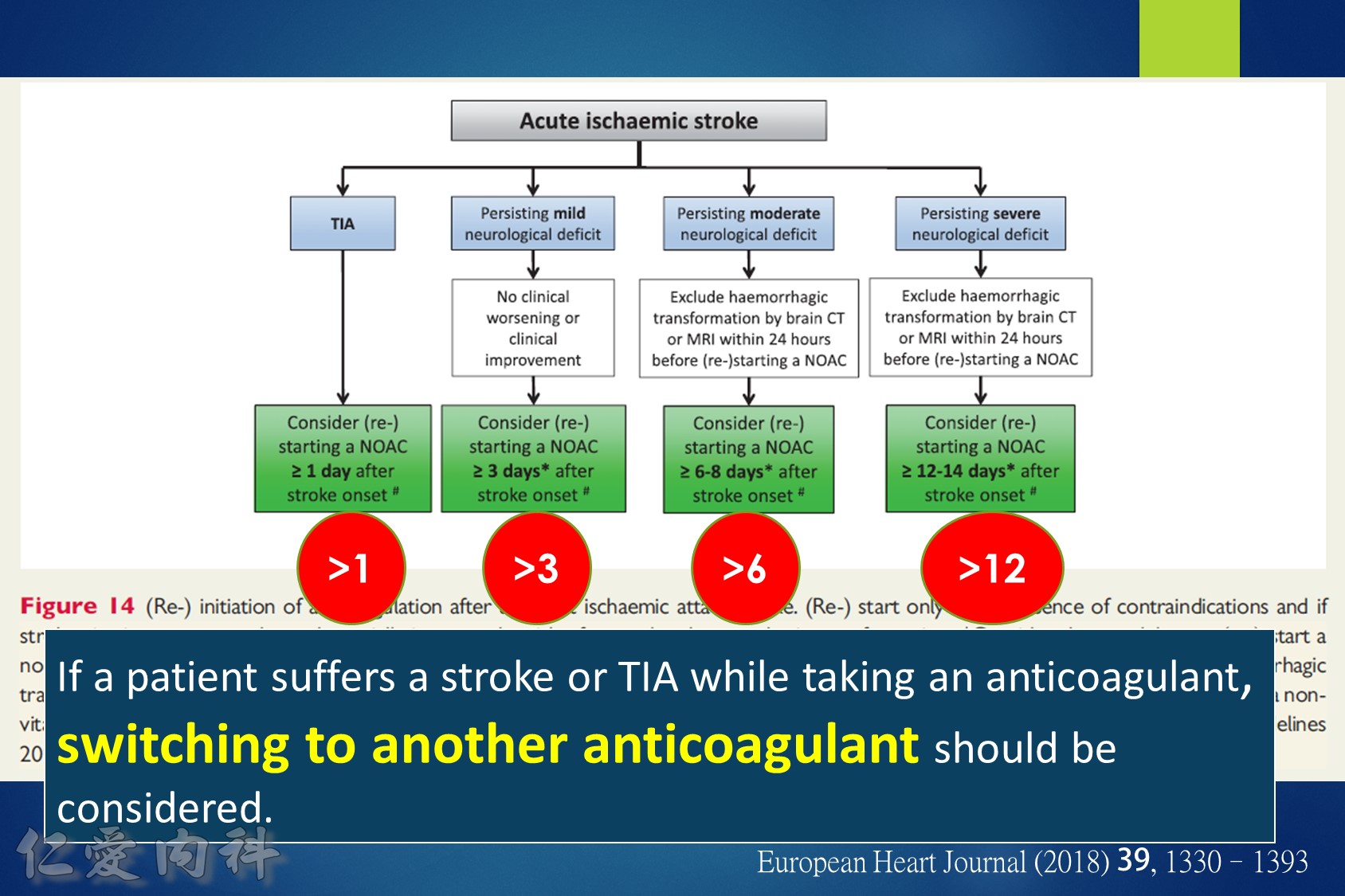
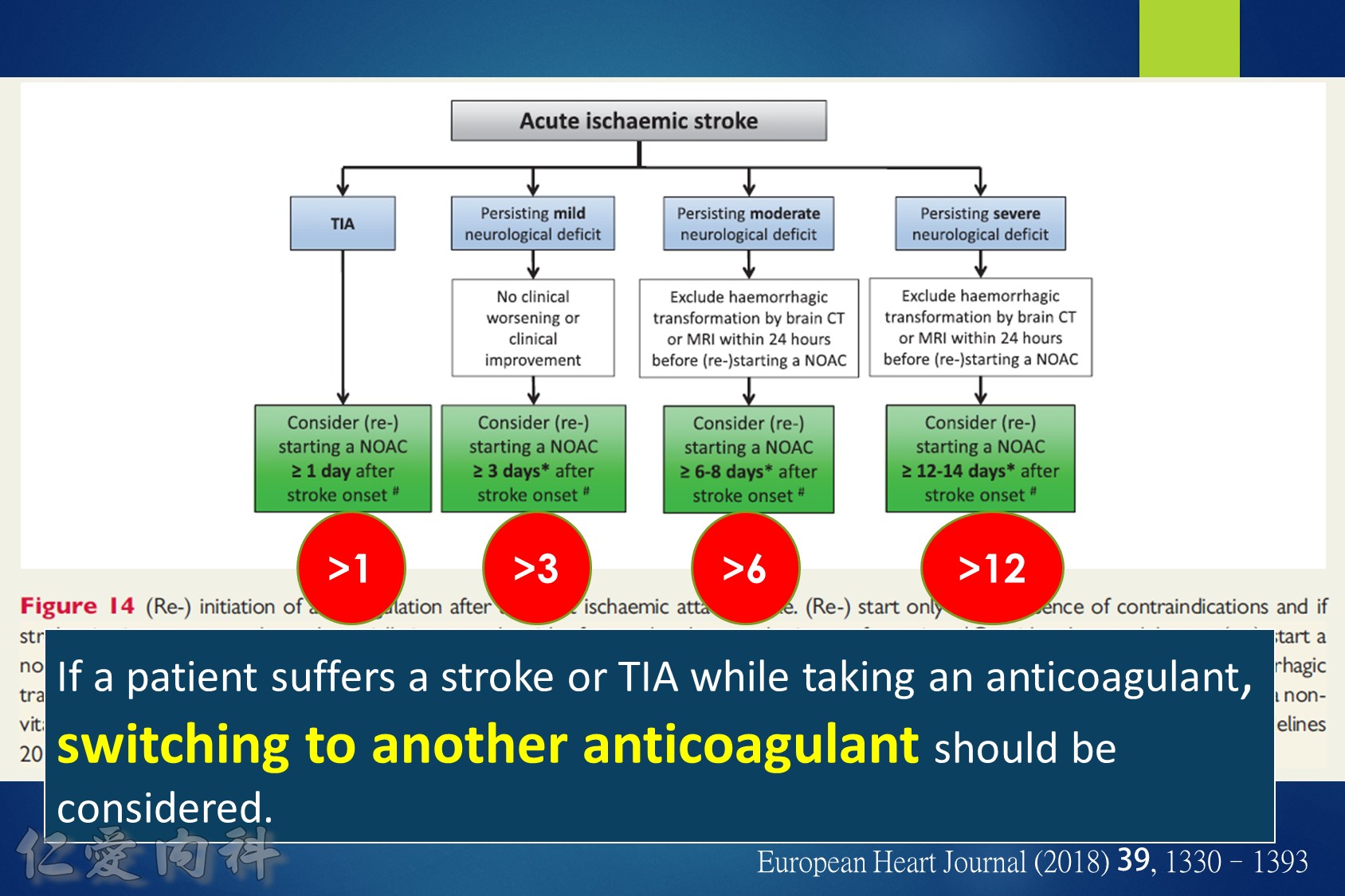
14.For patients taking NOACs who present with an acute ischemic stroke, consider re-starting NOACs after 3-14 days, depending on the degree of neurologic deficit and excluding any hemorrhagic transformation on brain computed tomography.
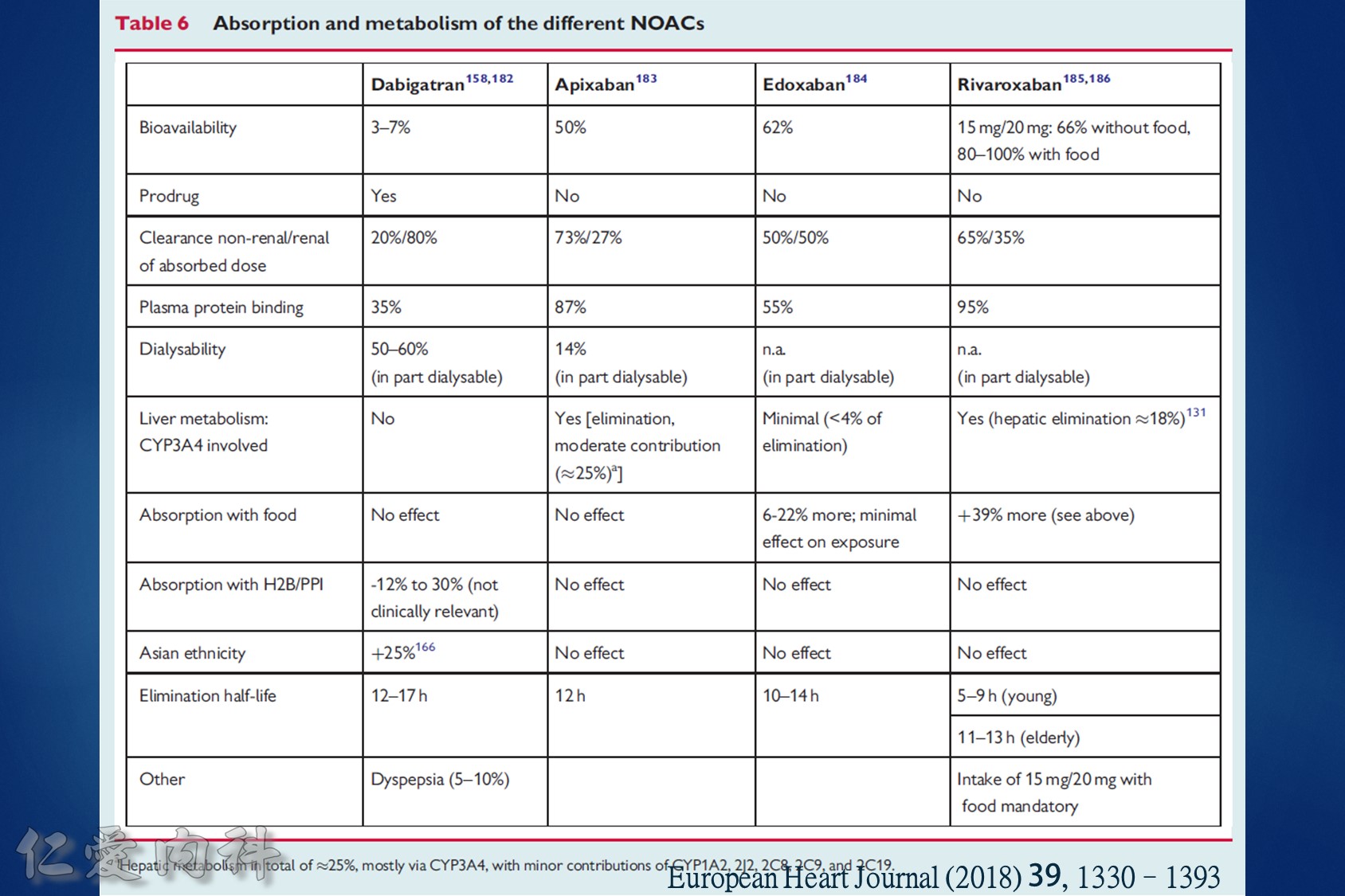
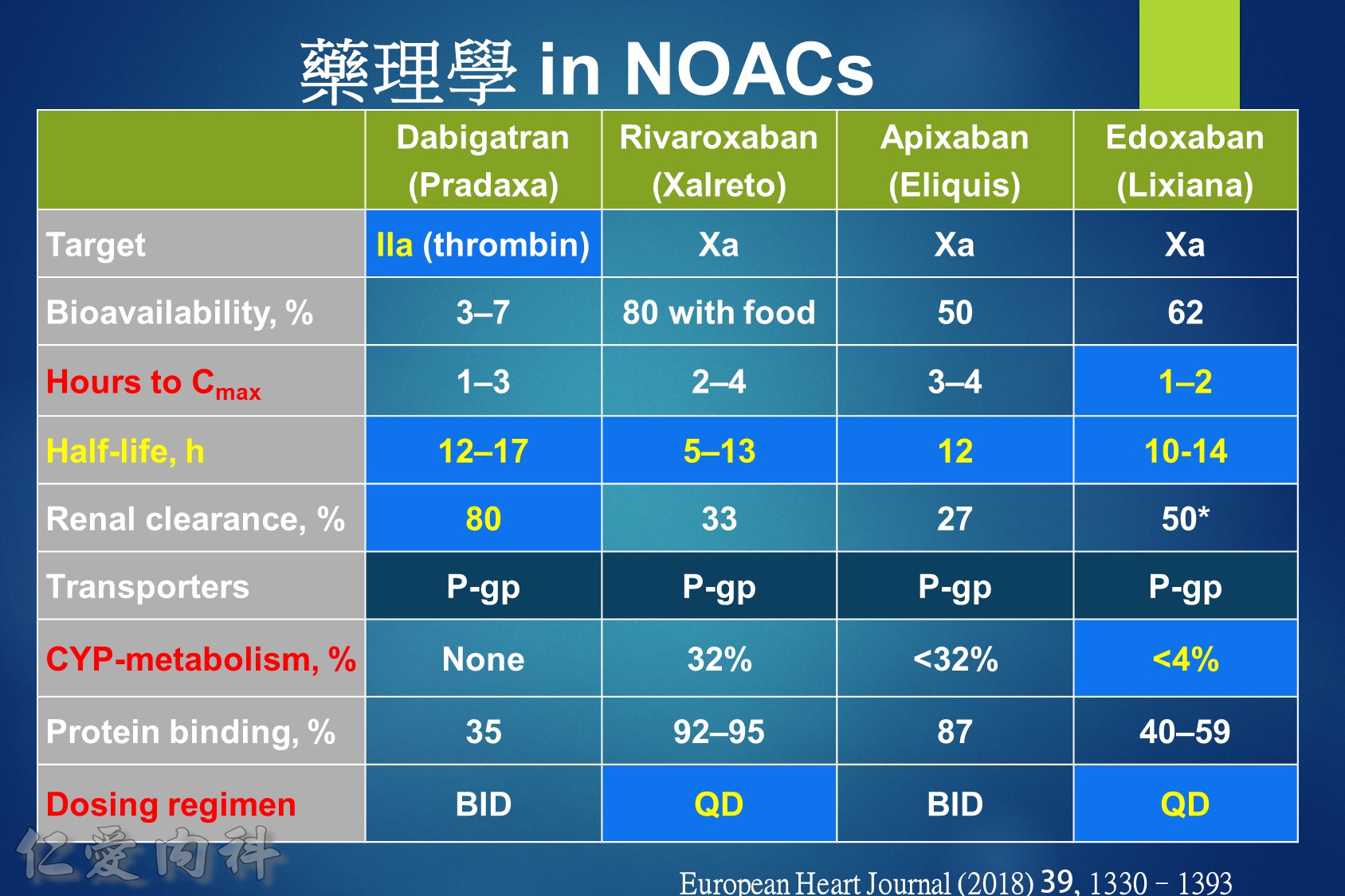
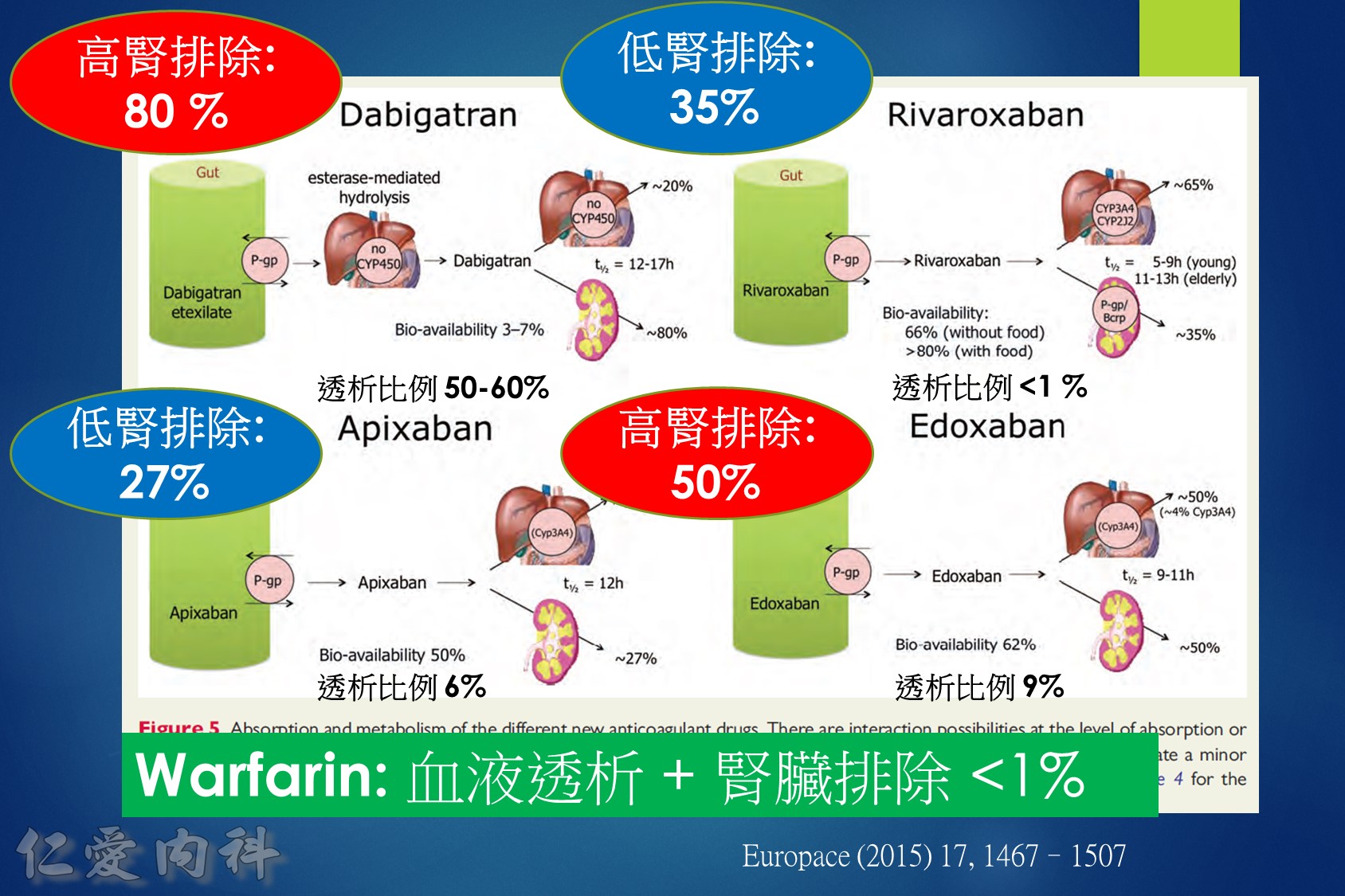
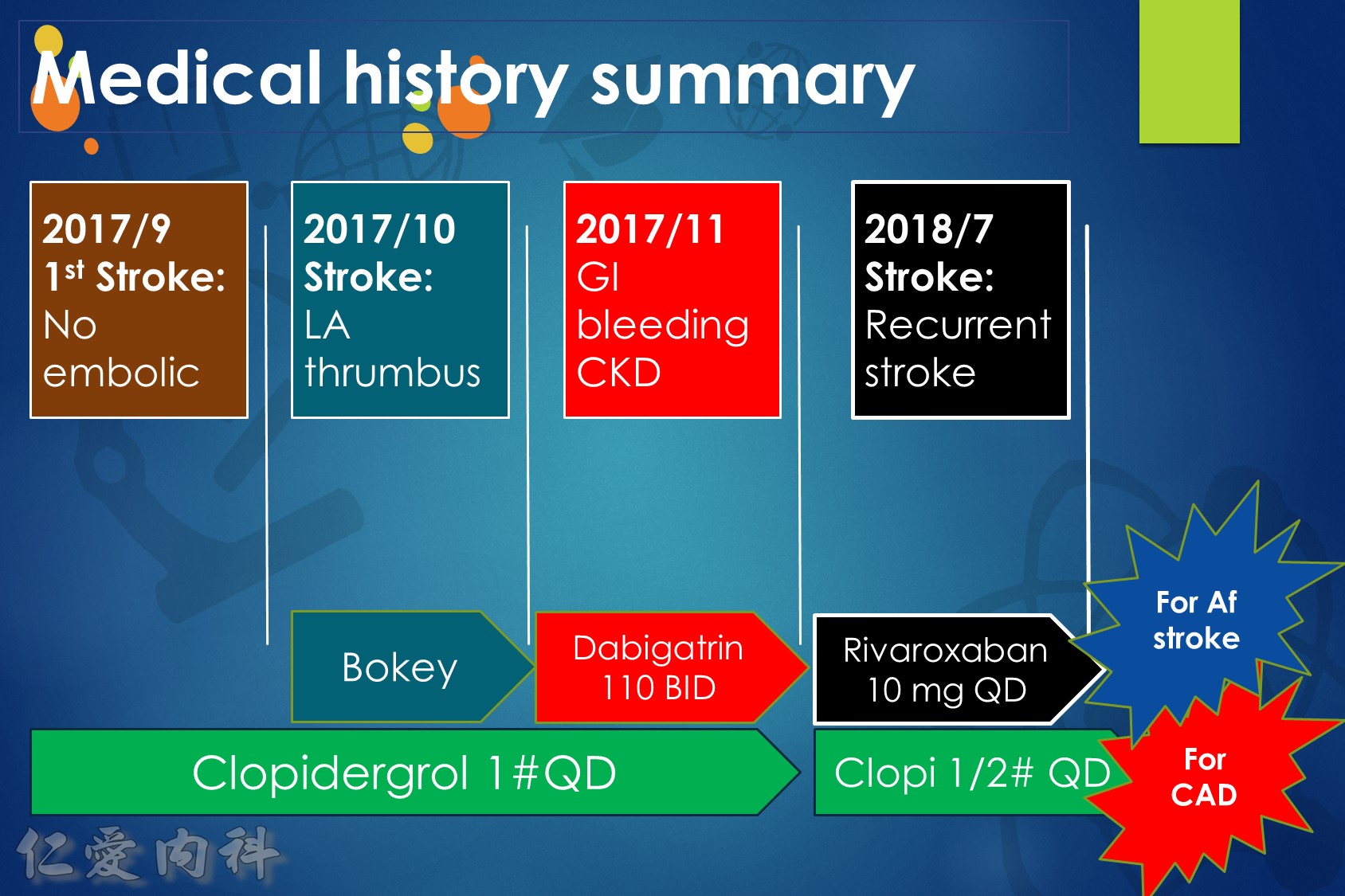
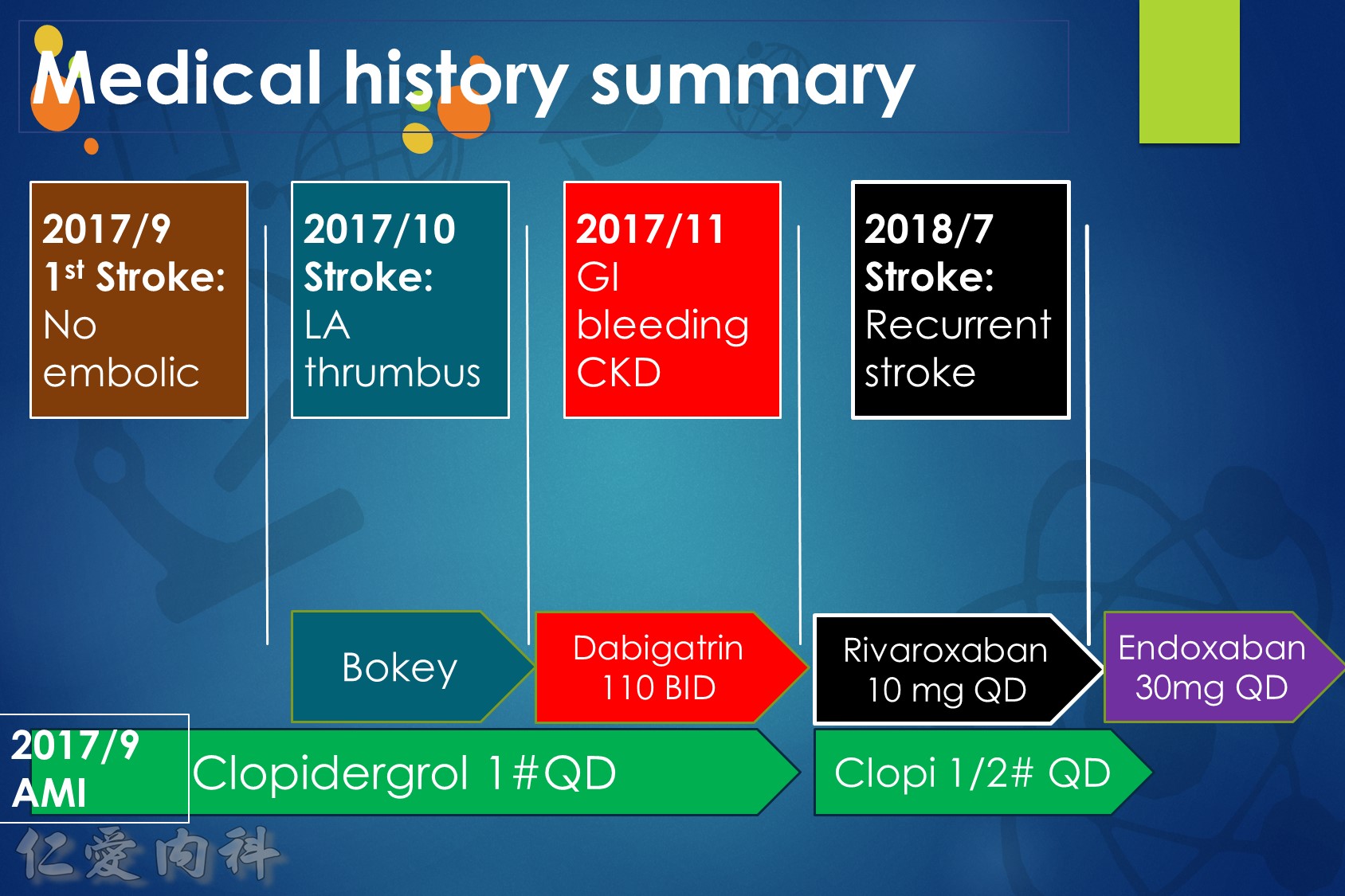
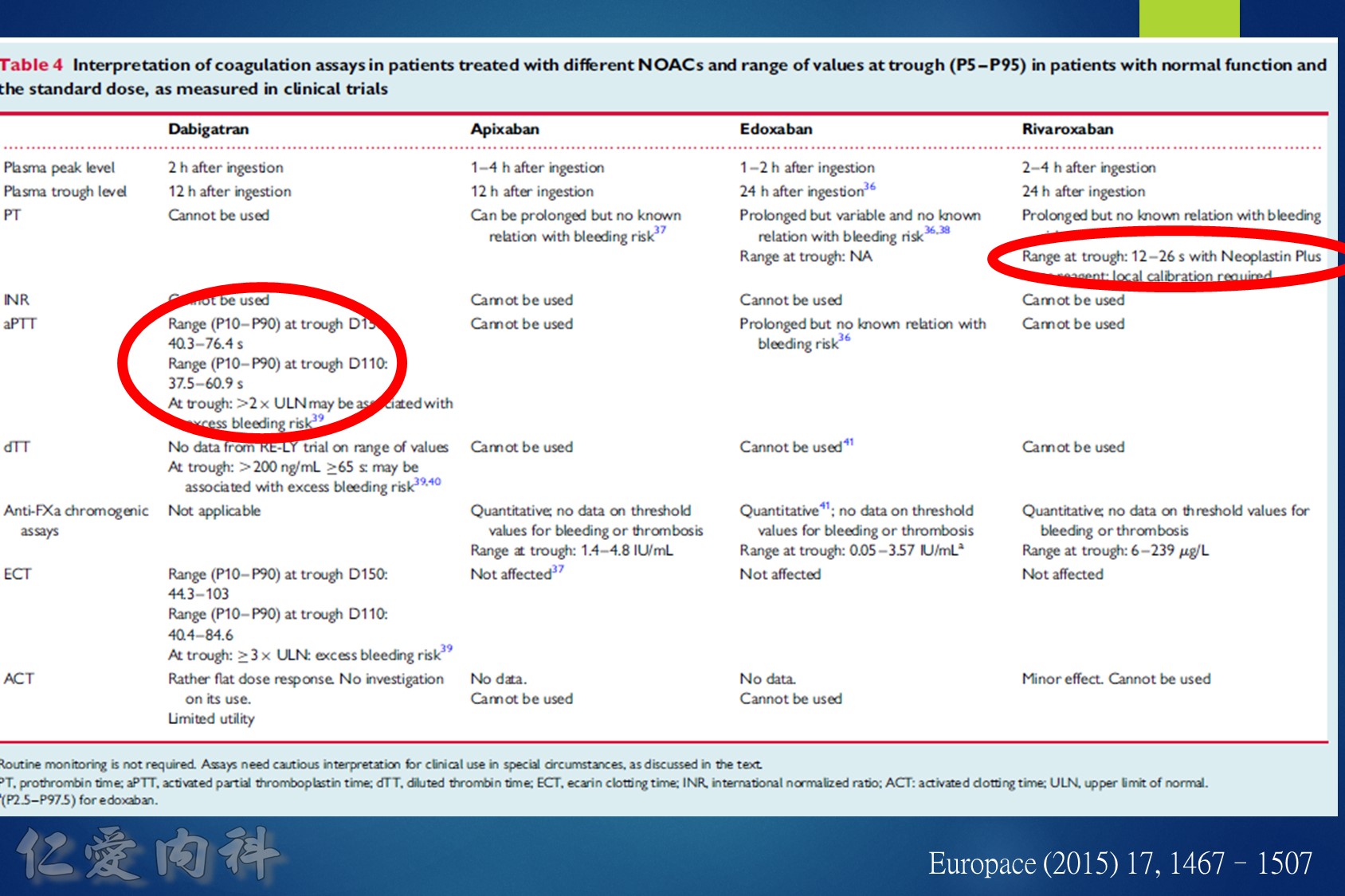
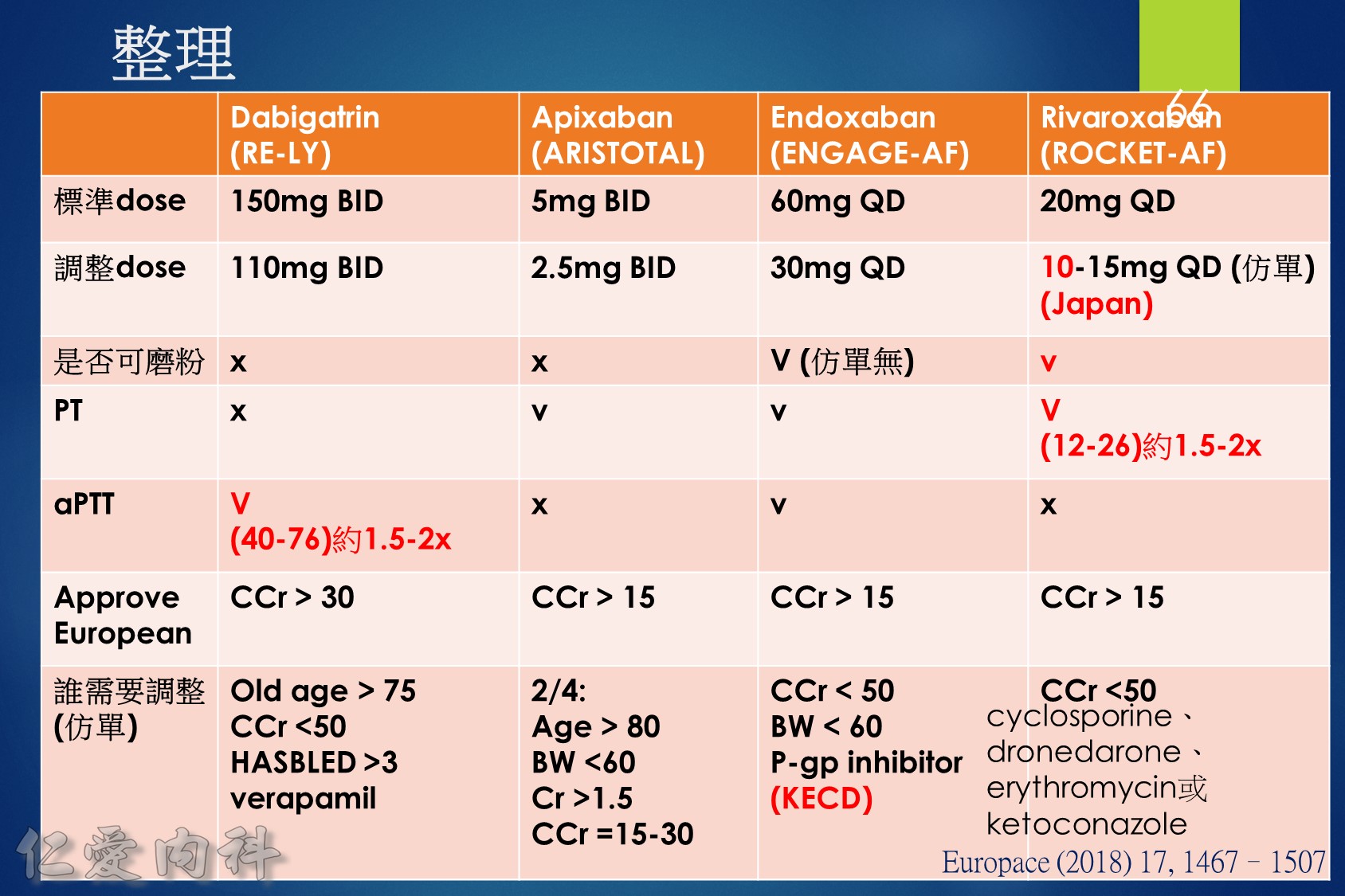
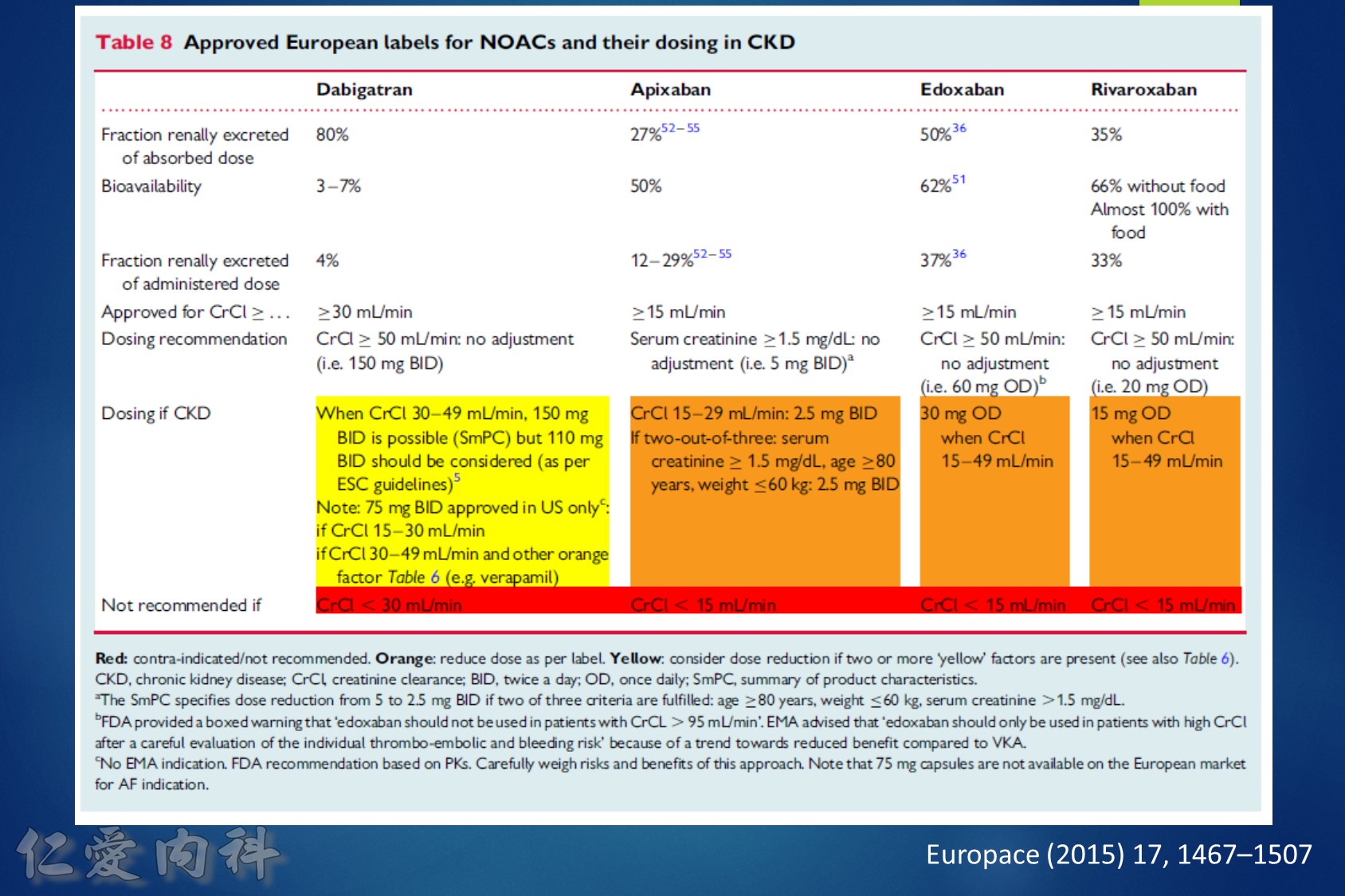
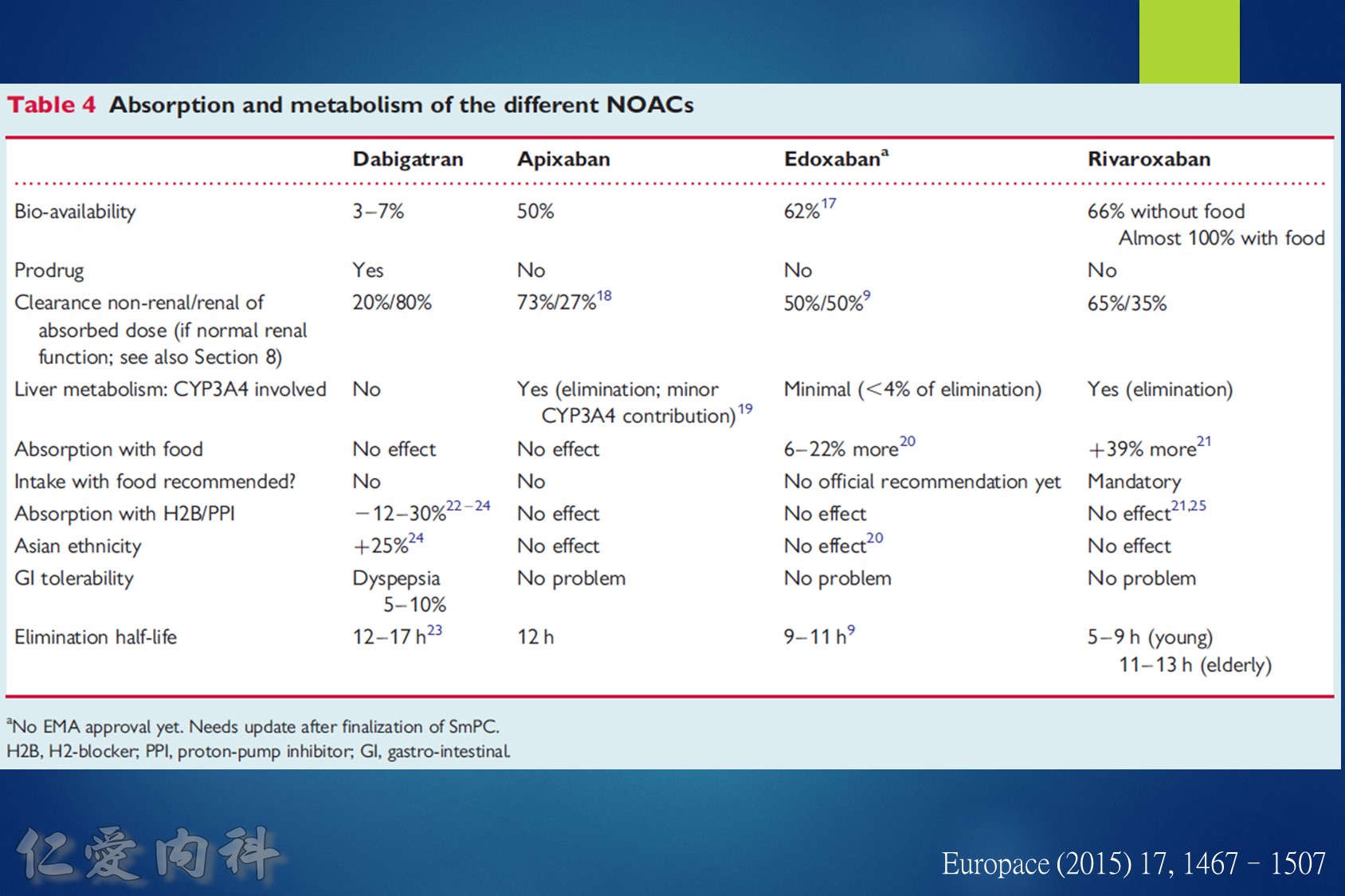
低腎臟排除:warfarin <1%、apixaban=27%、rivaroxaban=36%
中高腎臟排除:dabigatran=80%、edoxaban=50%
低血液透析排除:warfarin<1%、apixaban=7%、rivaroxaban<1%、edoxaban=9%
中高血液透析排除:dabigatran (50~60%)。
Drug choice for Af + Hemodialysis: Warfarin
至於腎功能肌酐酸清除率 15 mL/min 以上的病人,使用 rivaroxaban 與 dabigatran 並沒有增加重大出血 (major bleeding) 風險 (與使用 warfarin 者比較)。至於肌肝酸清除率15 mL/min以下的心房顫動病人,US FDA 的建議是可以使用 initial 5 mg BID 的 apixaban。另外,給予腎功能不全的病人使用 apixaban 與 edoxaban,重大出血風險有顯著降低 (相較於warfarin)。
發生抗凝血劑相關之ICH後,何時可在使用上抗凝血劑? (2015 AHA)
- Avoidance of oral anticoagulation for at least 4 weeks, in patients without mechanical heart valves, might decrease the risk of ICH recurrence (Class IIb; Level of Evidence B). (New recommendation)
- If indicated, aspirin monotherapy can probably be restarted in the days after ICH, although the optimal timing is uncertain (Class IIa; Level of Evidence B). (New recommendation)
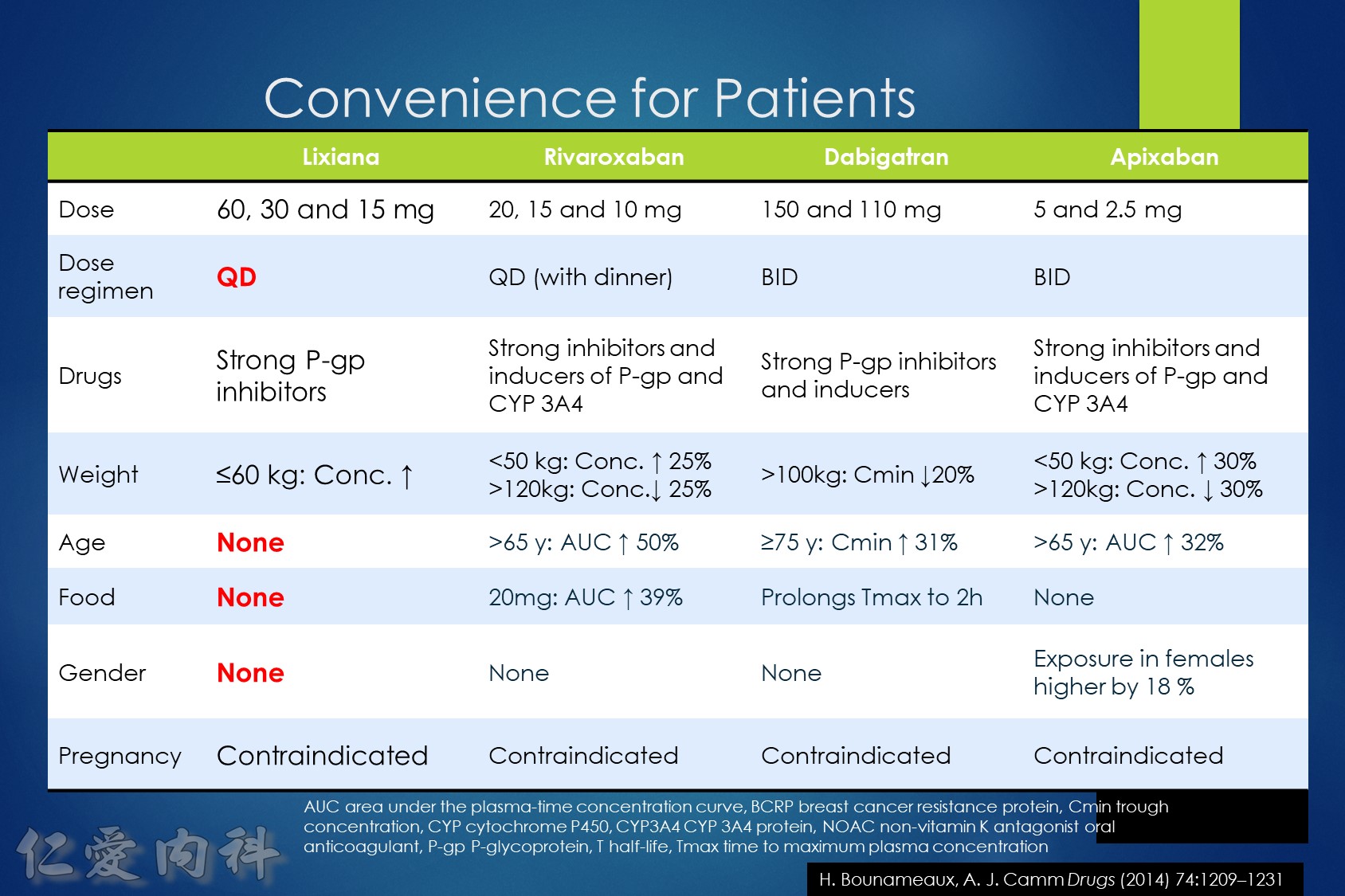
Lixiana 是 P 醣蛋白 (P-gp) 外排運輸蛋白的受質。藥物動力學研究顯示,Lixiana 若與 cyclosporine、dronedarone、erythromycin、ketoconazole、quinidine 或verapamil 等 P 醣蛋白 (P-gp) 抑制劑併用,會導致 Lixiana 的血漿濃度上升。如 Lixiana 併用 cyclosporine、dronedarone、erythromycin 或 ketoconazole,必須將劑量調降成每日一次30毫克。
(Ref: 藥品仿單)
Summary
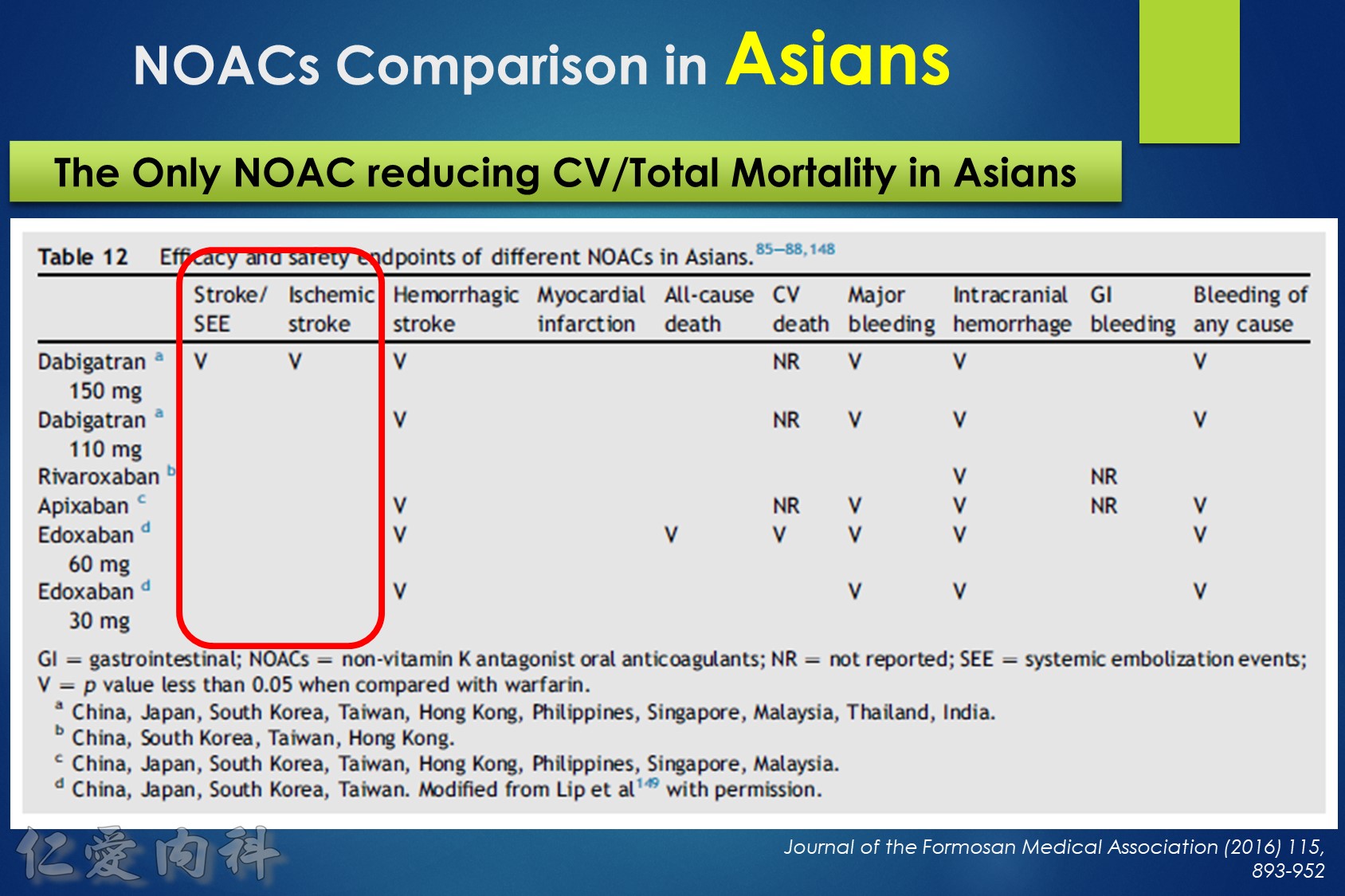
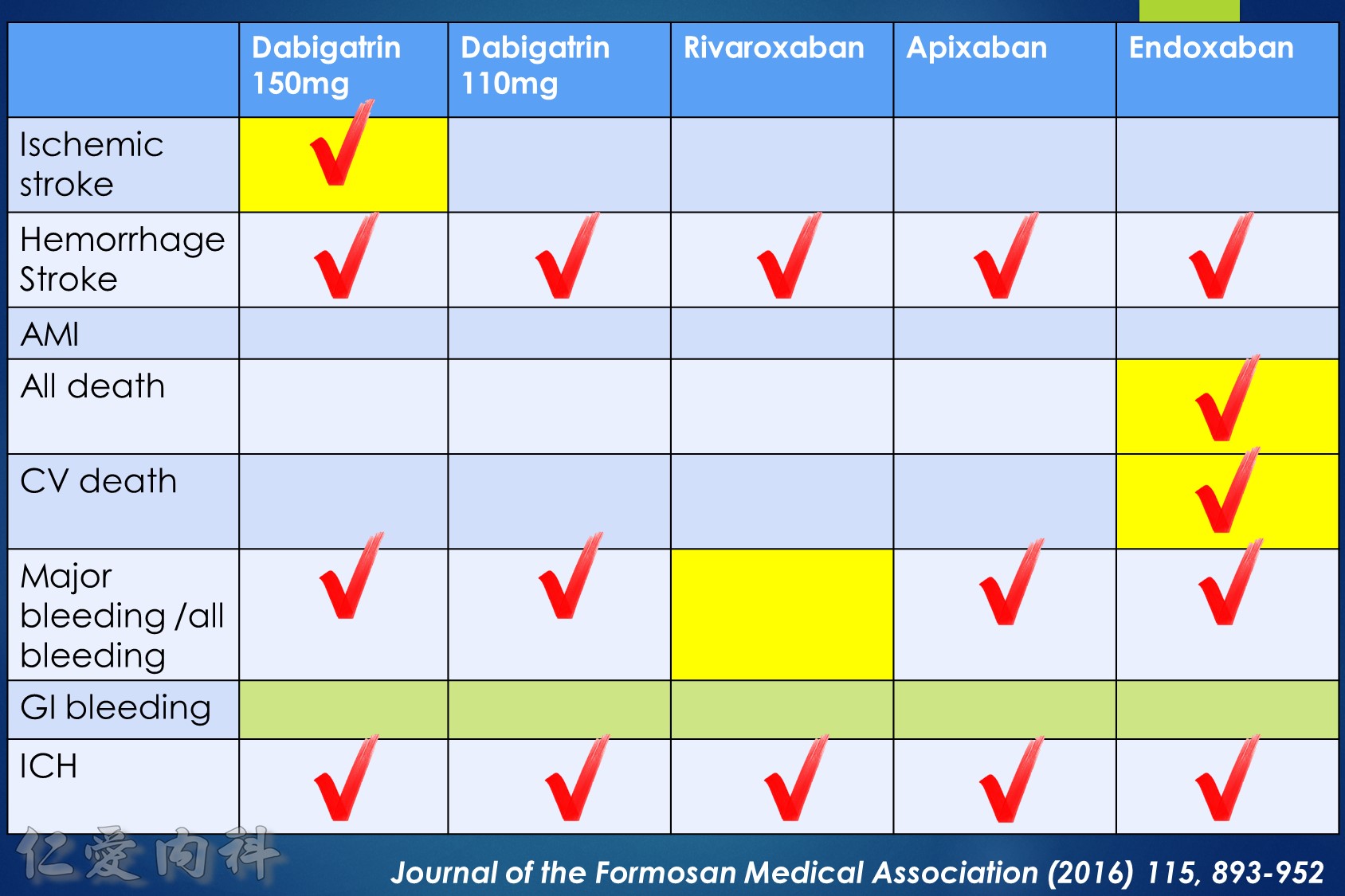
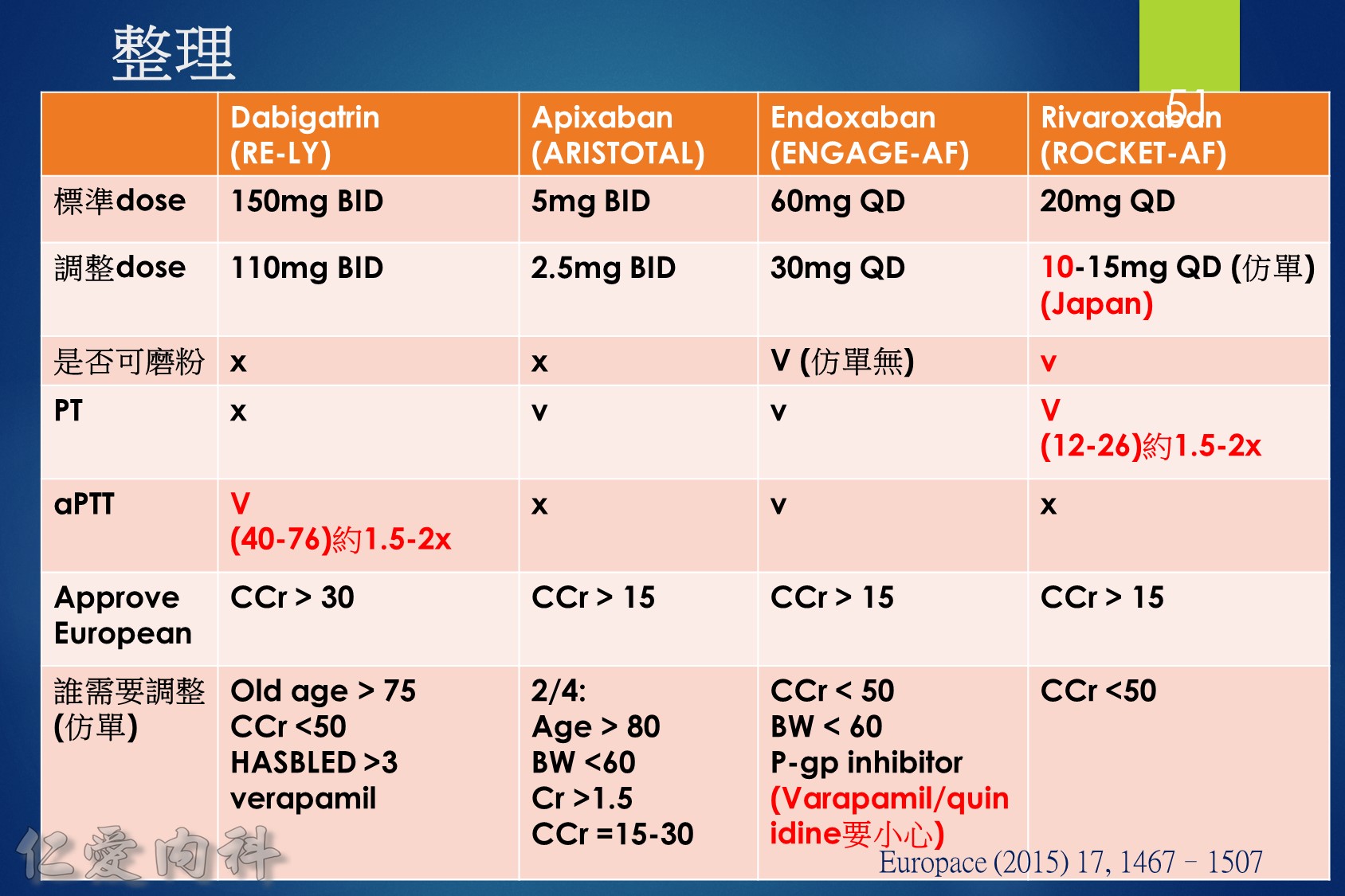
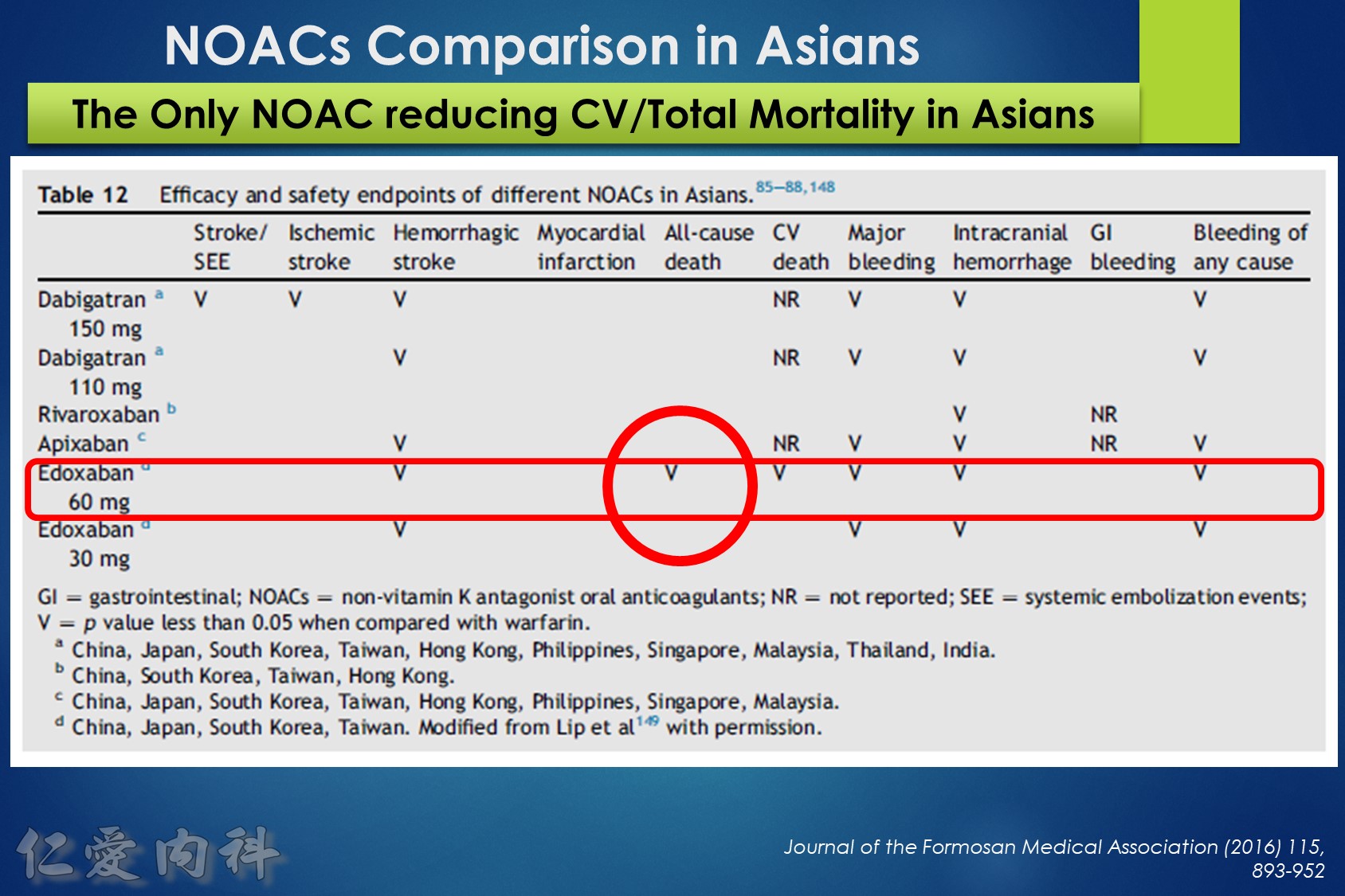
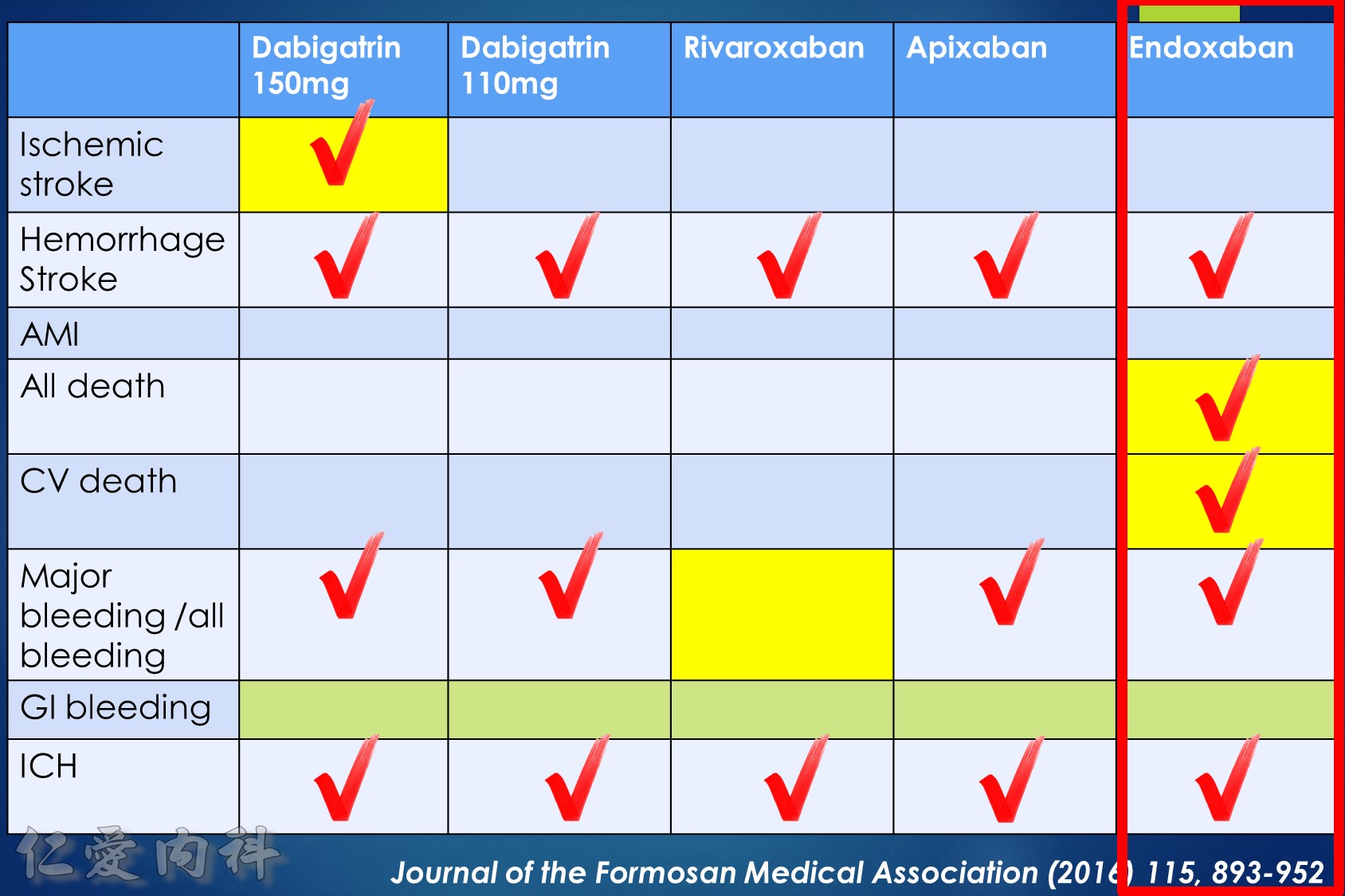
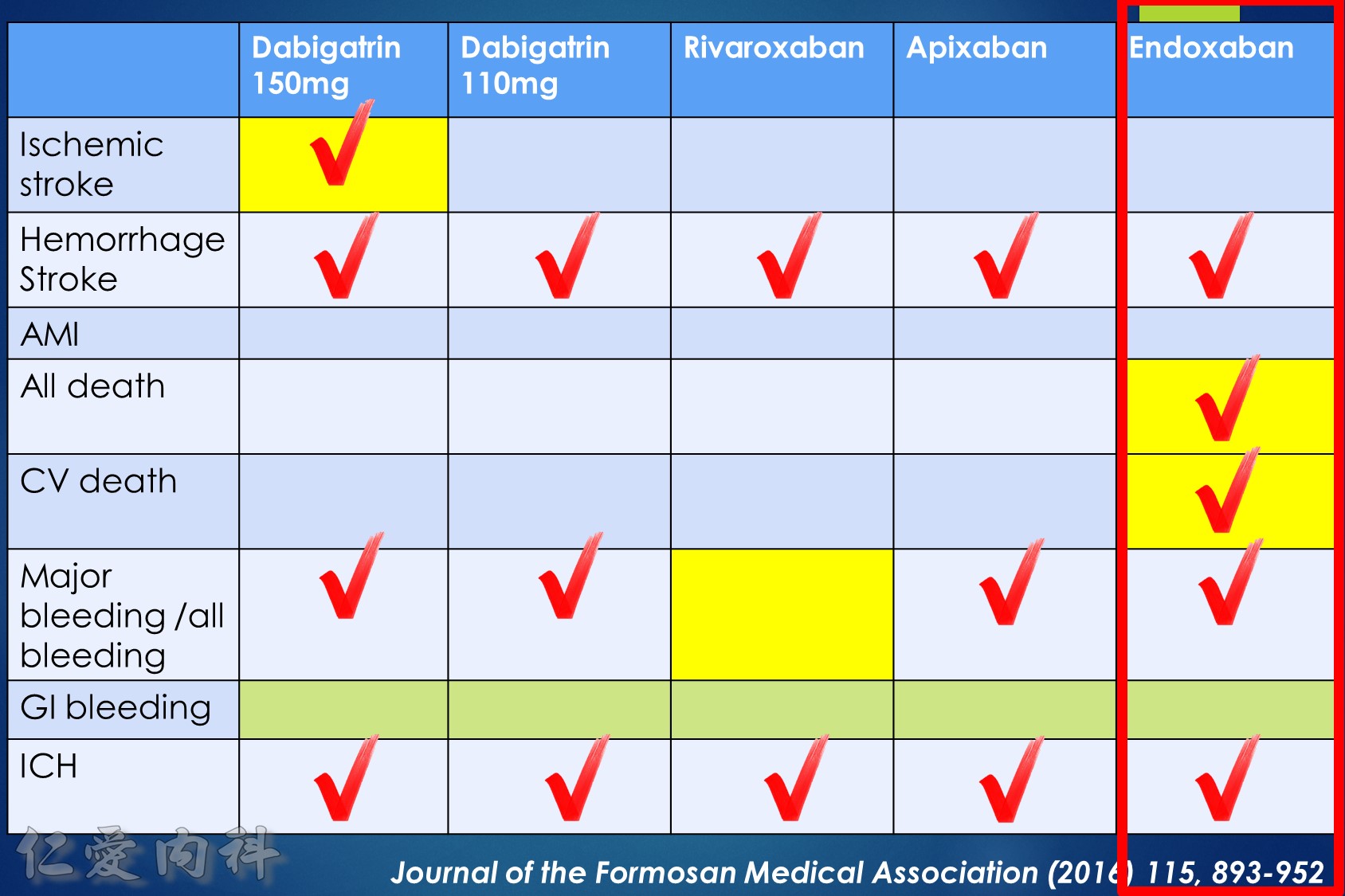
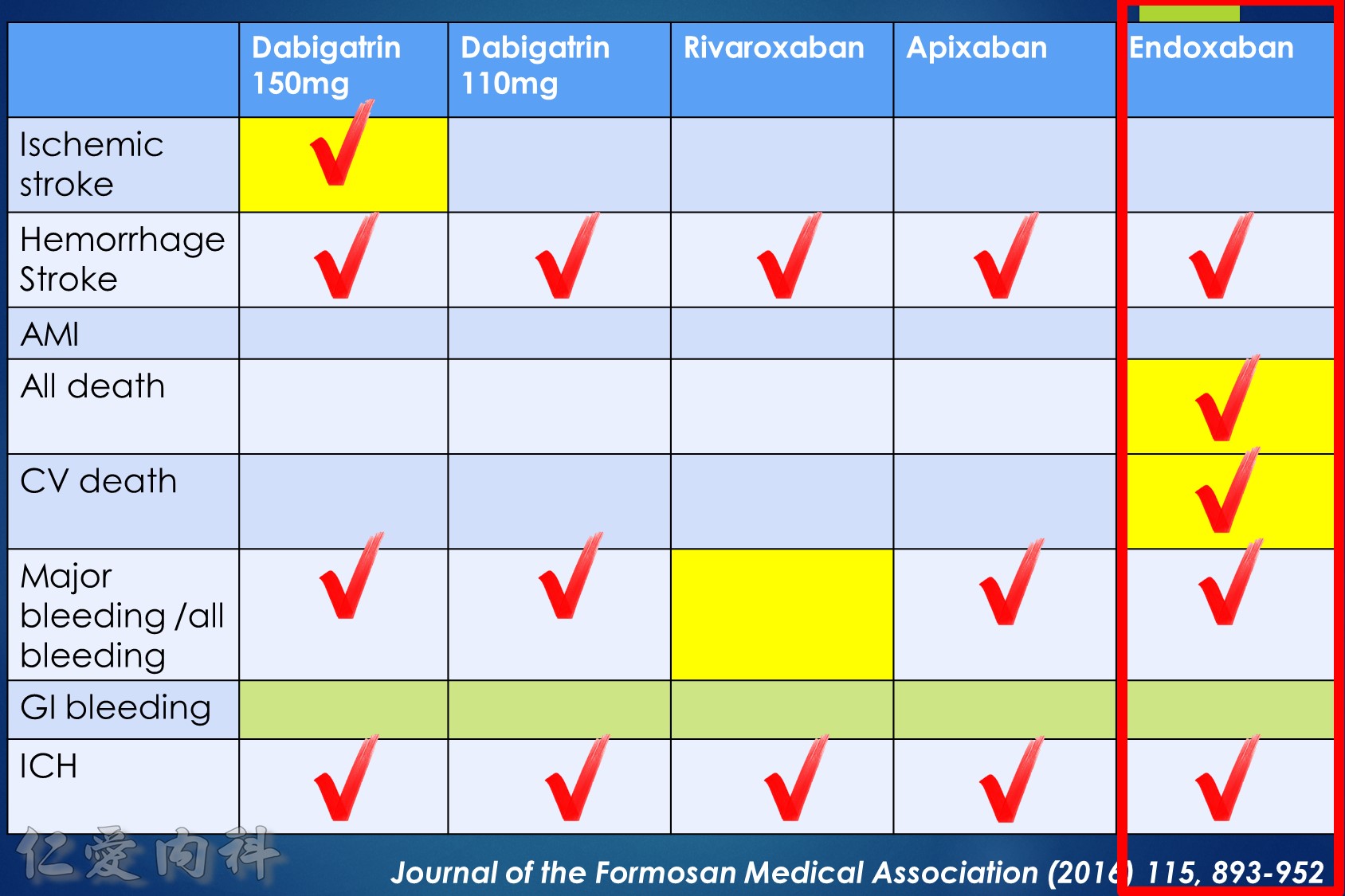
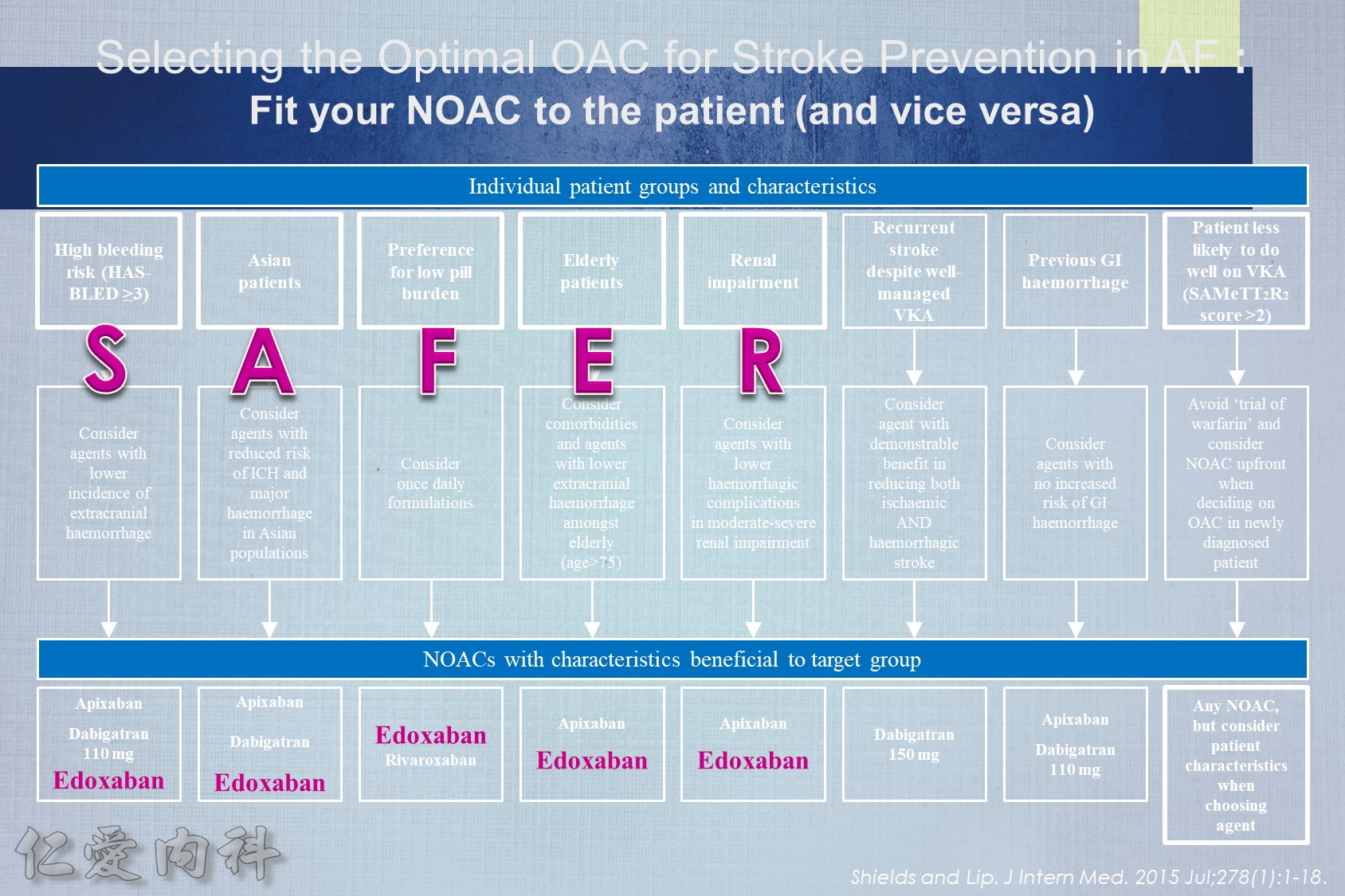
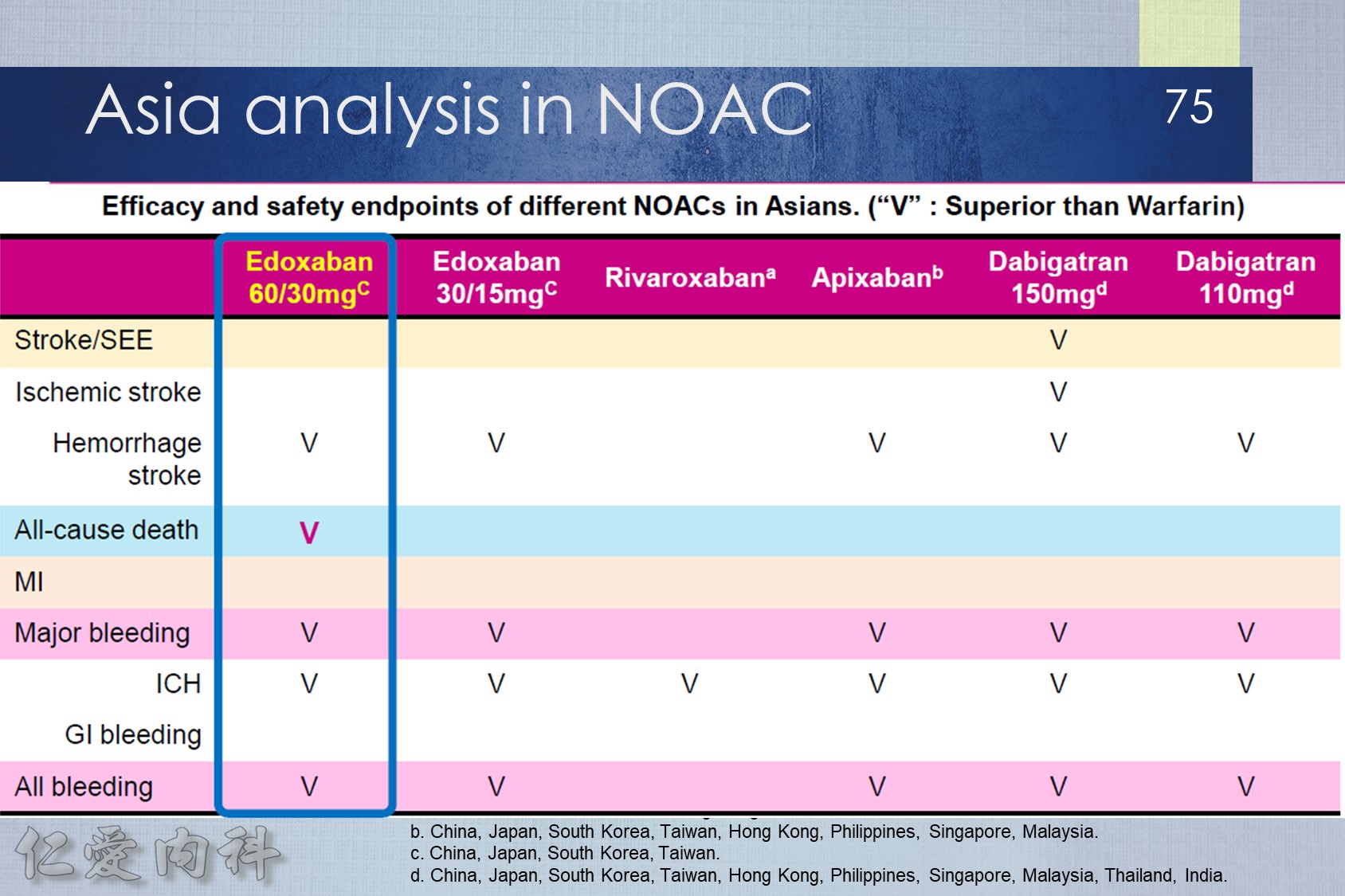
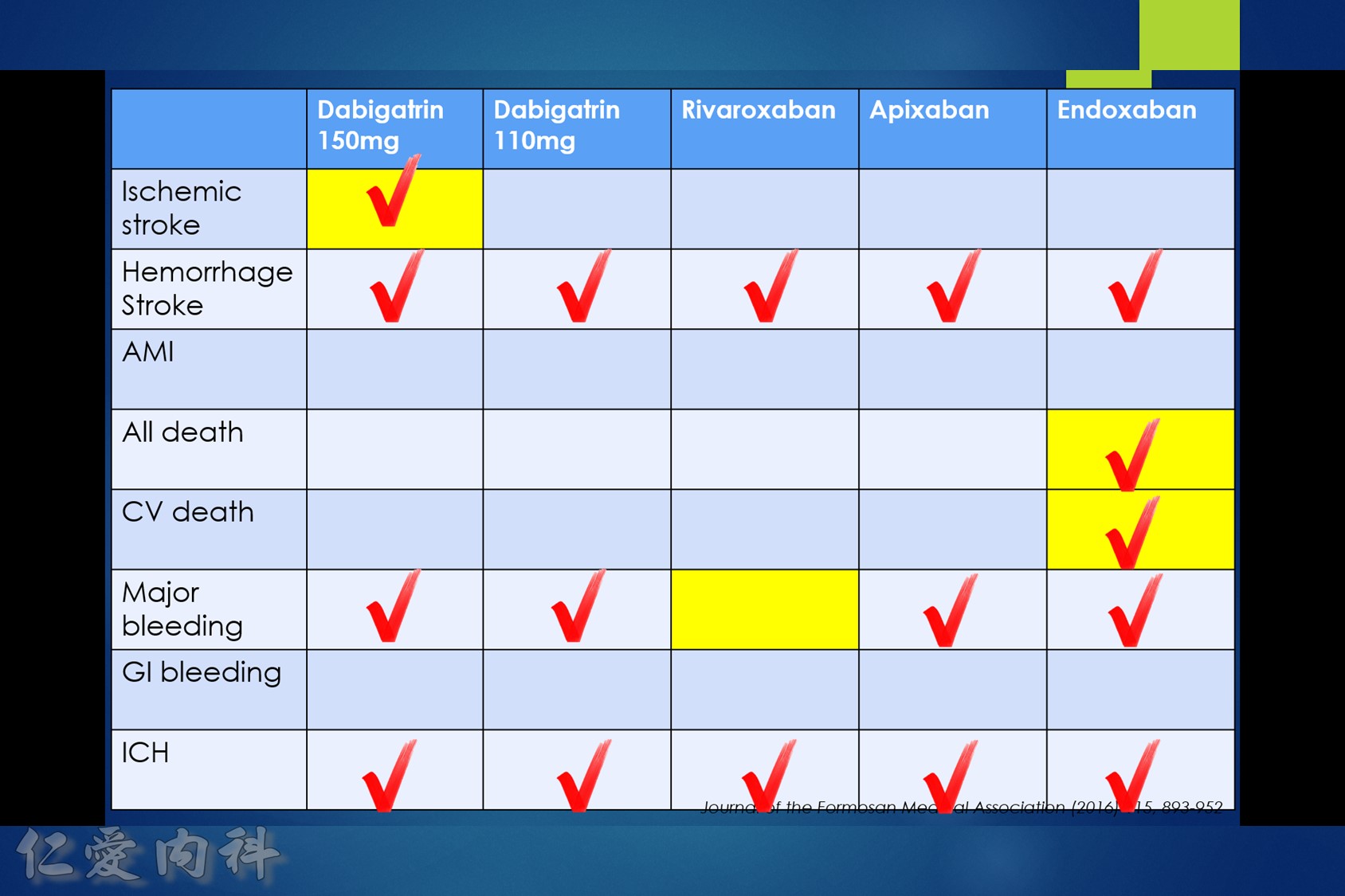
- NOAC 選擇需做個人化之調整
- Pradaxa 150 mg 對於預防中風較好
- Edoxaban 60/30 可以降低 mortality
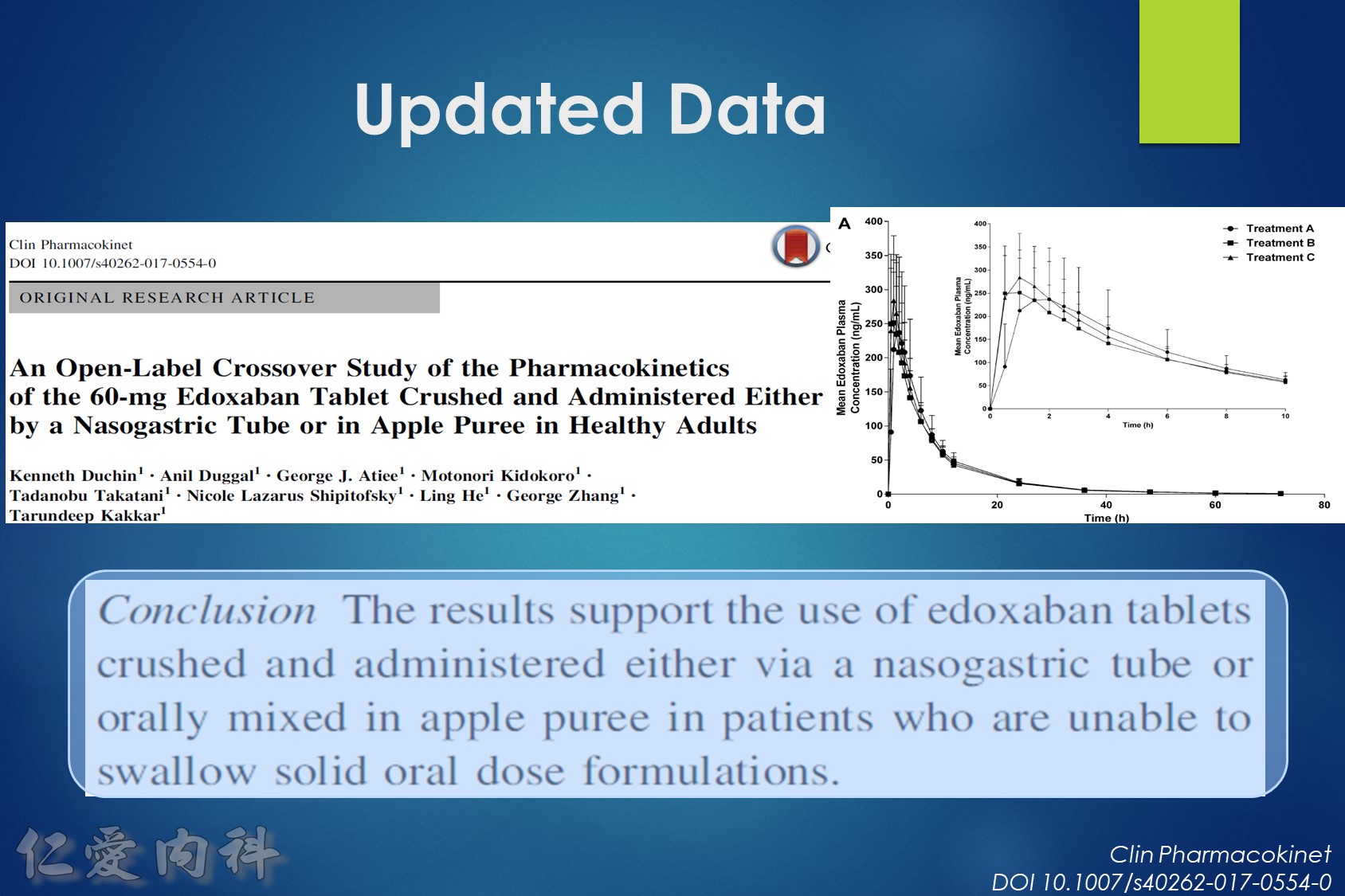
- 可磨粉? Rivaroxaban / Edoxaban (?)
- 注意腎功能以及是否有嚴重瓣膜問題 (機械瓣膜 or severe MS)
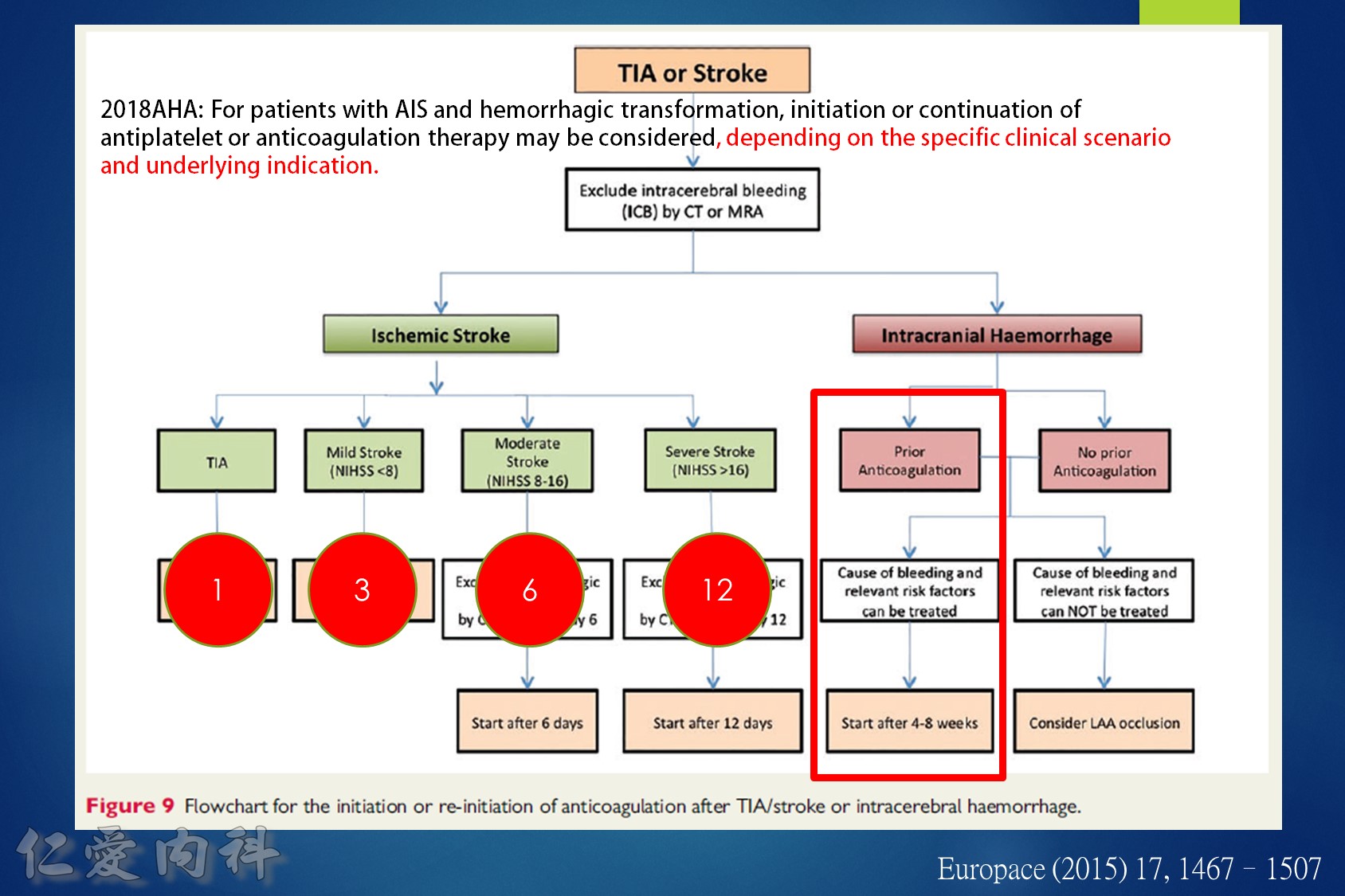
- 中風後 anti-coagulation 可以參考 1-3-6-12 rule
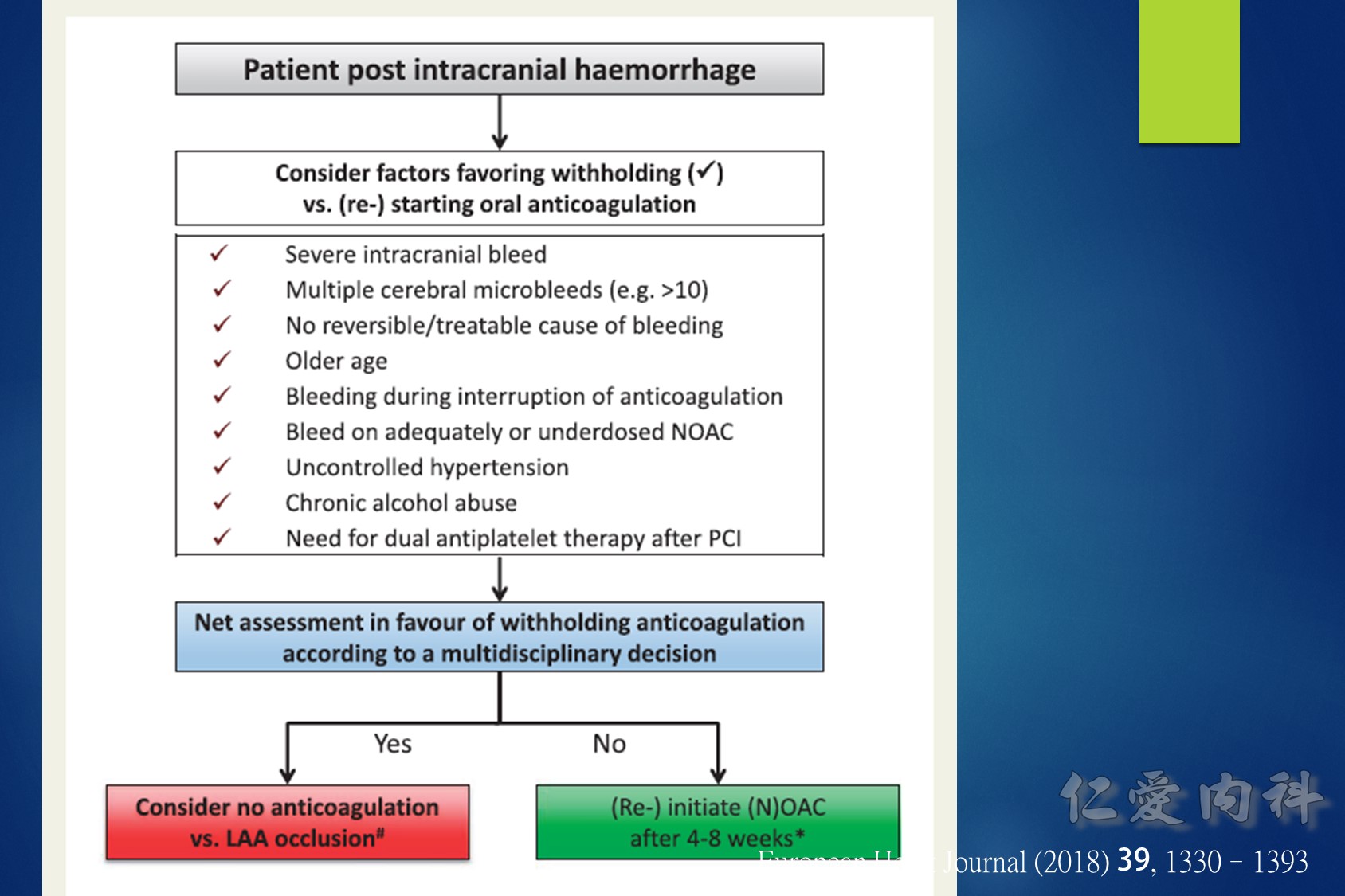
- ICH 後 4-8 周可以加回藥物
- 加回藥物前記得要做 brain CT
- AMI + PCI 後: 剛開始用 NOAC + Anti-PLT
- Low bleeding risk: 12 months 後可單用 NOAC
- High bleeding risk: 6 months 後可單用 NOAC
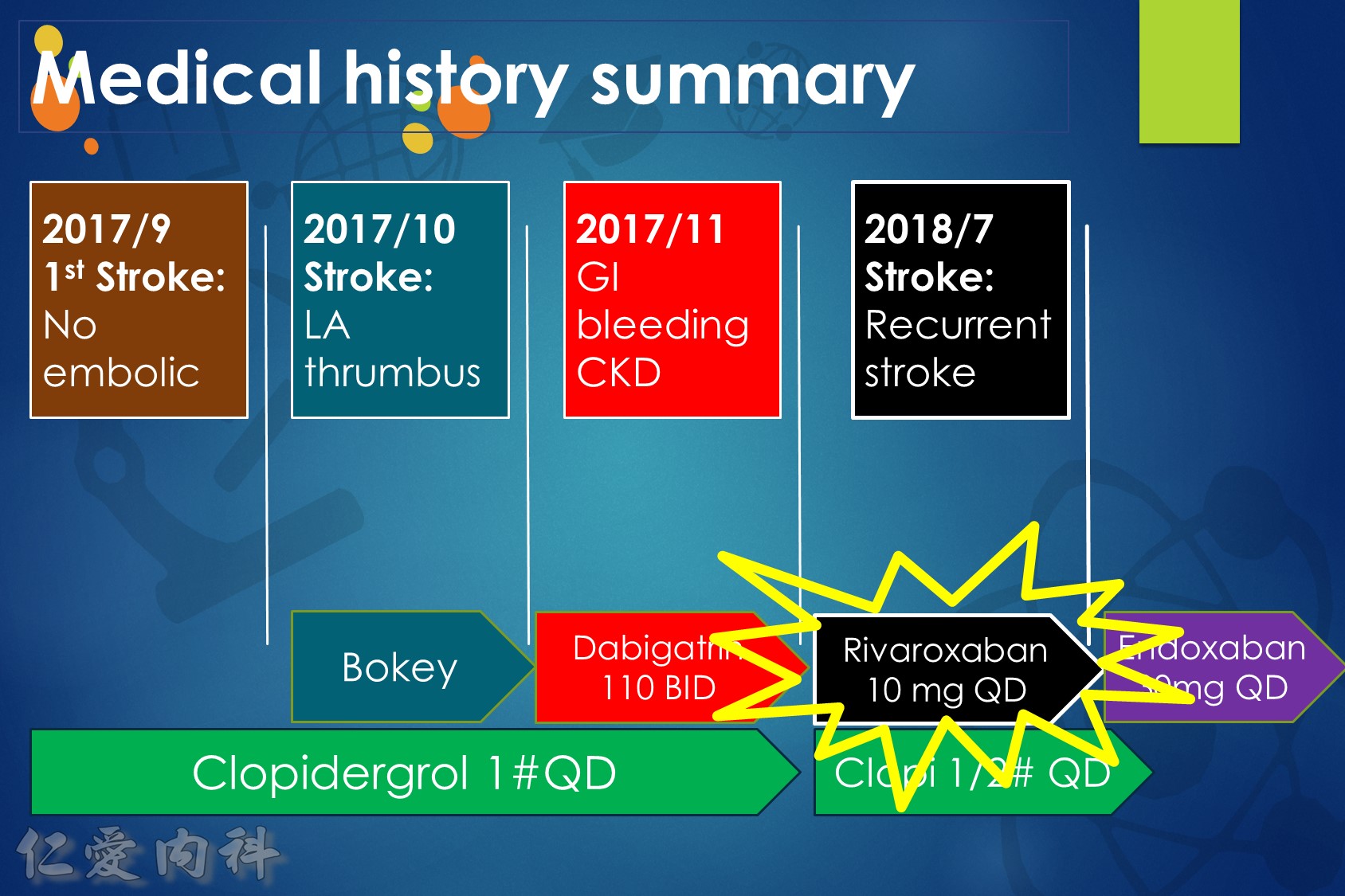
Some advocate as a rule of thumb the 1–3–6–12 day rule, with re-institution of anticoagulation in patients with a transient ischaemic attack (TIA) after 1 day, with small, non-disabling infarct after 3 days, with a moderate stroke after 6 days, while large infarcts involving large parts of the arterial territory will be treated not before 2 (or even 3) weeks. (Heidbuchel et al., Paragraph 14.2.2)
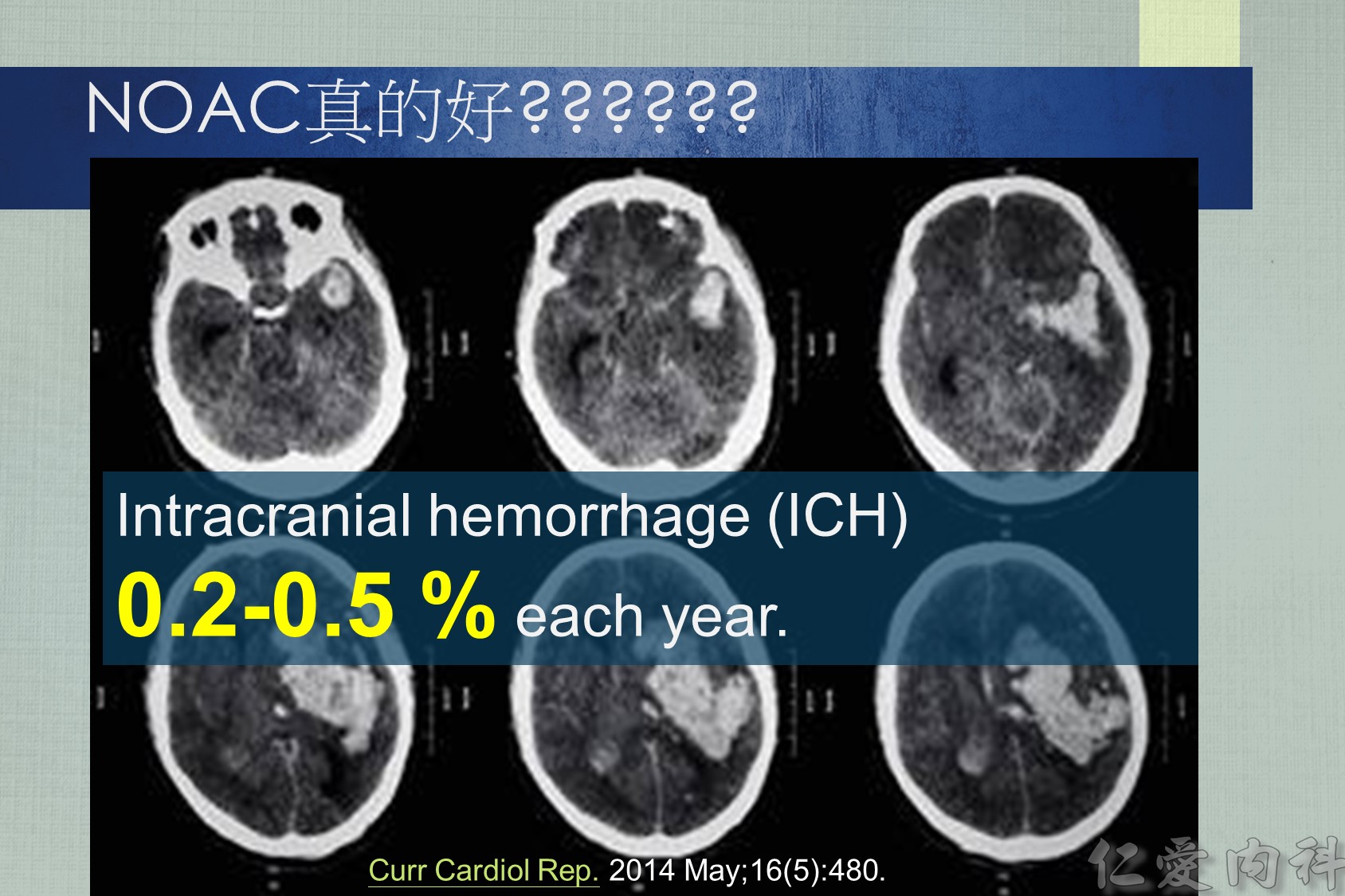
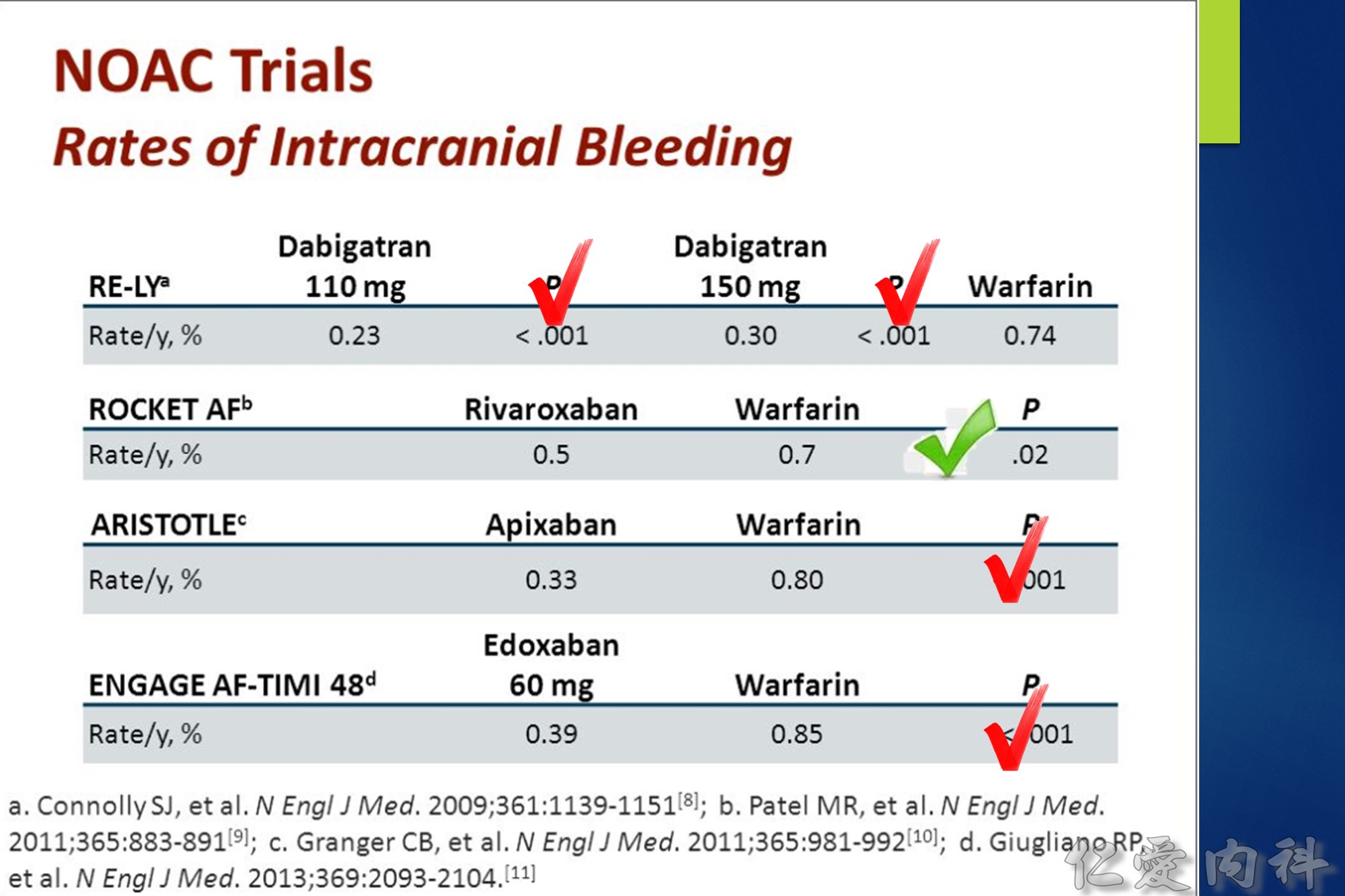
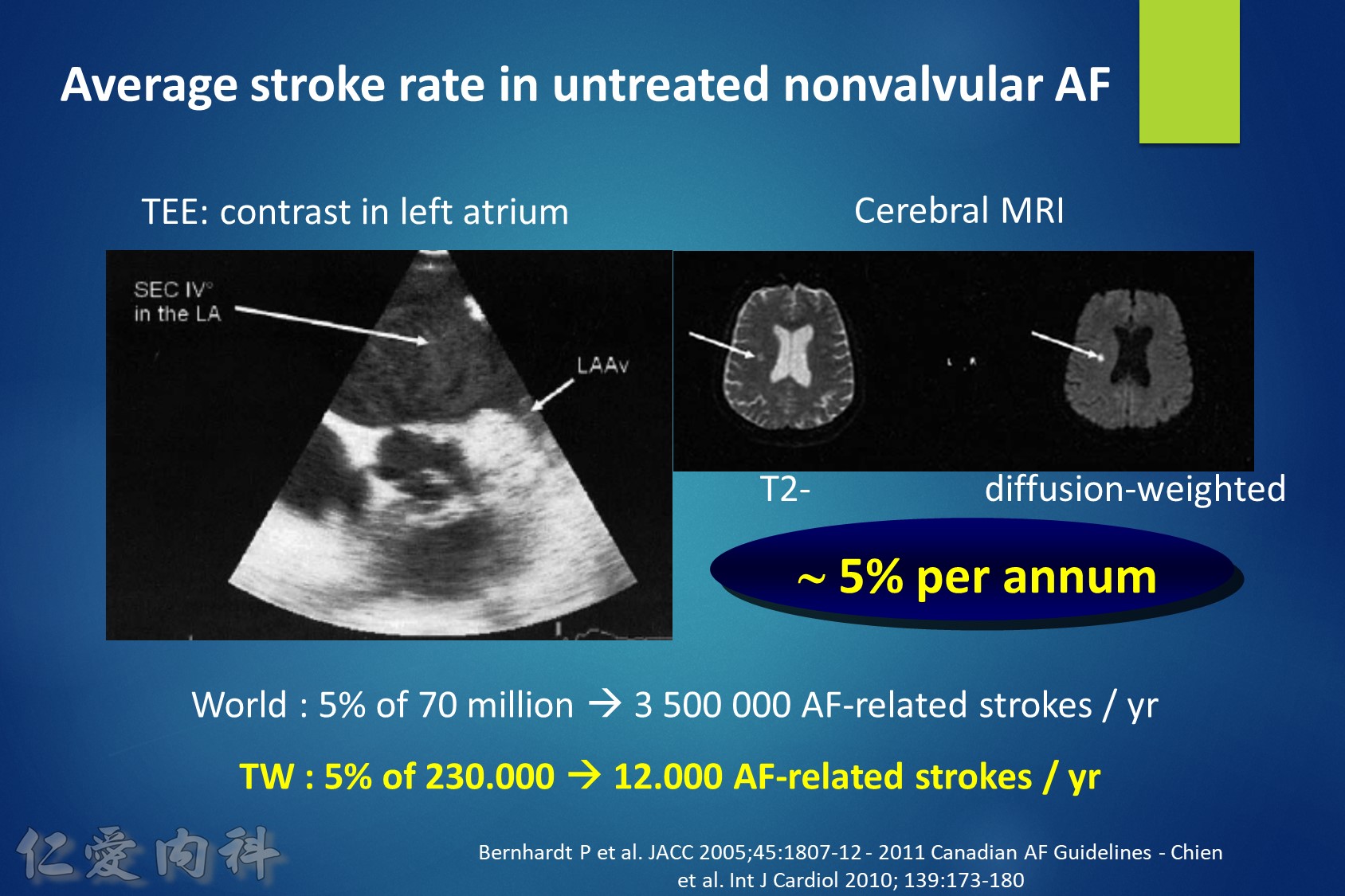
Intracranial hemorrhage (ICH) affects 0.2-0.5 % of atrial fibrillation (AF) patients taking a novel oral anticoagulant (NOAC) each year. About two thirds of ICHs are intracerebral and one quarter subdural. The 30-day case fatality of NOAC-associated ICH was similar to that of warfarin-associated ICH in two trials. Consistent predictors of ICH are increasing age, a history of prior stroke or TIA, and concomitant use of an antiplatelet drug. Compared to warfarin, the NOACs significantly reduce the risk of ICH by half (risk ratio = 0.44; 95 % CI: 0.37 to 0.51). Compared to aspirin, apixaban has a similar risk of ICH (risk ratio = 0.84; 95 % CI, 0.38 to 1.87). Current treatments for NOAC-associated ICH include nonactivated and activated prothrombin complex concentrate, which reverse the anticoagulant effects of the NOACs, but their effects on bleeding and patient outcome are not known. Future treatments for NOAC-associated ICH promise to include specific antidotes to dabigatran (e.g., aDabi-Fab, PER977) and factor Xa inhibitors (e.g., r-Antidote PRT064445, PER977).
PMID: 24643903 DOI: 10.1007/s11886-014-0480-9
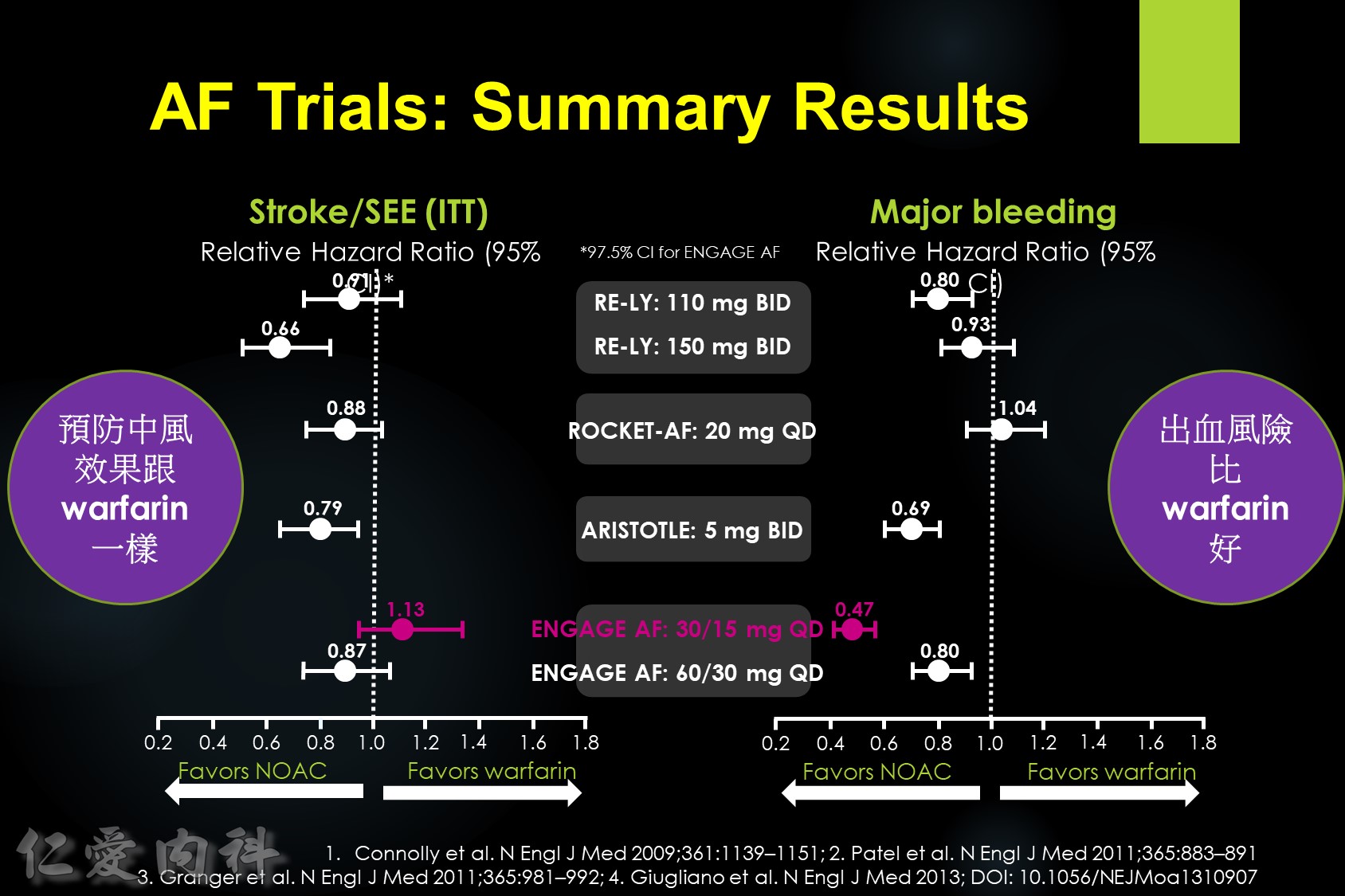
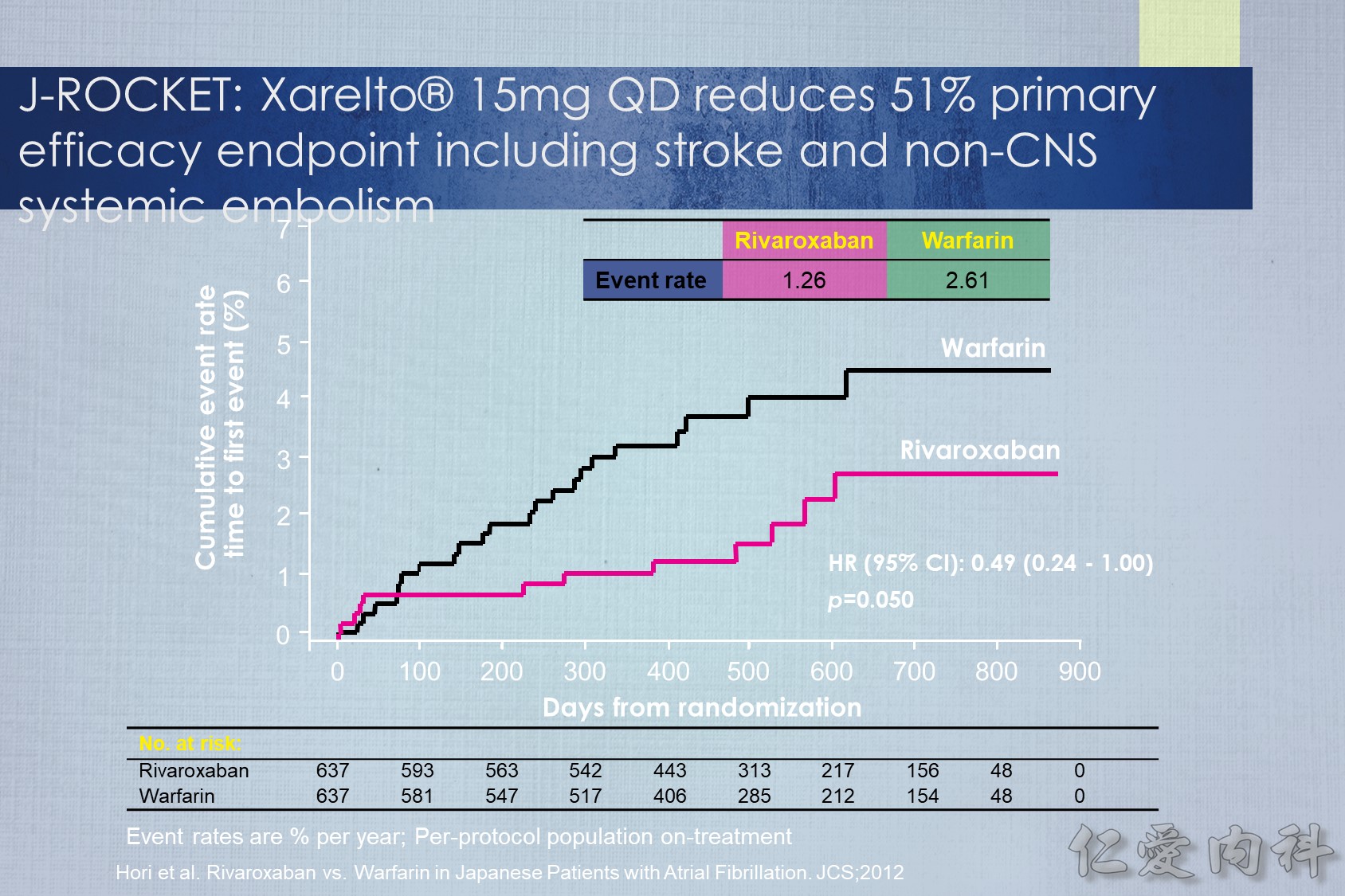
J-ROCKET AF primary efficacy endpoint
The primary efficacy endpoint of stroke and non-CNS systemic embolism was evaluated in the J-ROCKET AF study, although this study was not powered for efficacy. The Kaplan–Meier curves separated early and remained so. The hazard ratio for the primary efficacy endpoint was 0.49. There was a strong trend towards improved efficacy with rivaroxaban.
Reference
Hori M. et al. Circ J. 2012 Jun 5.
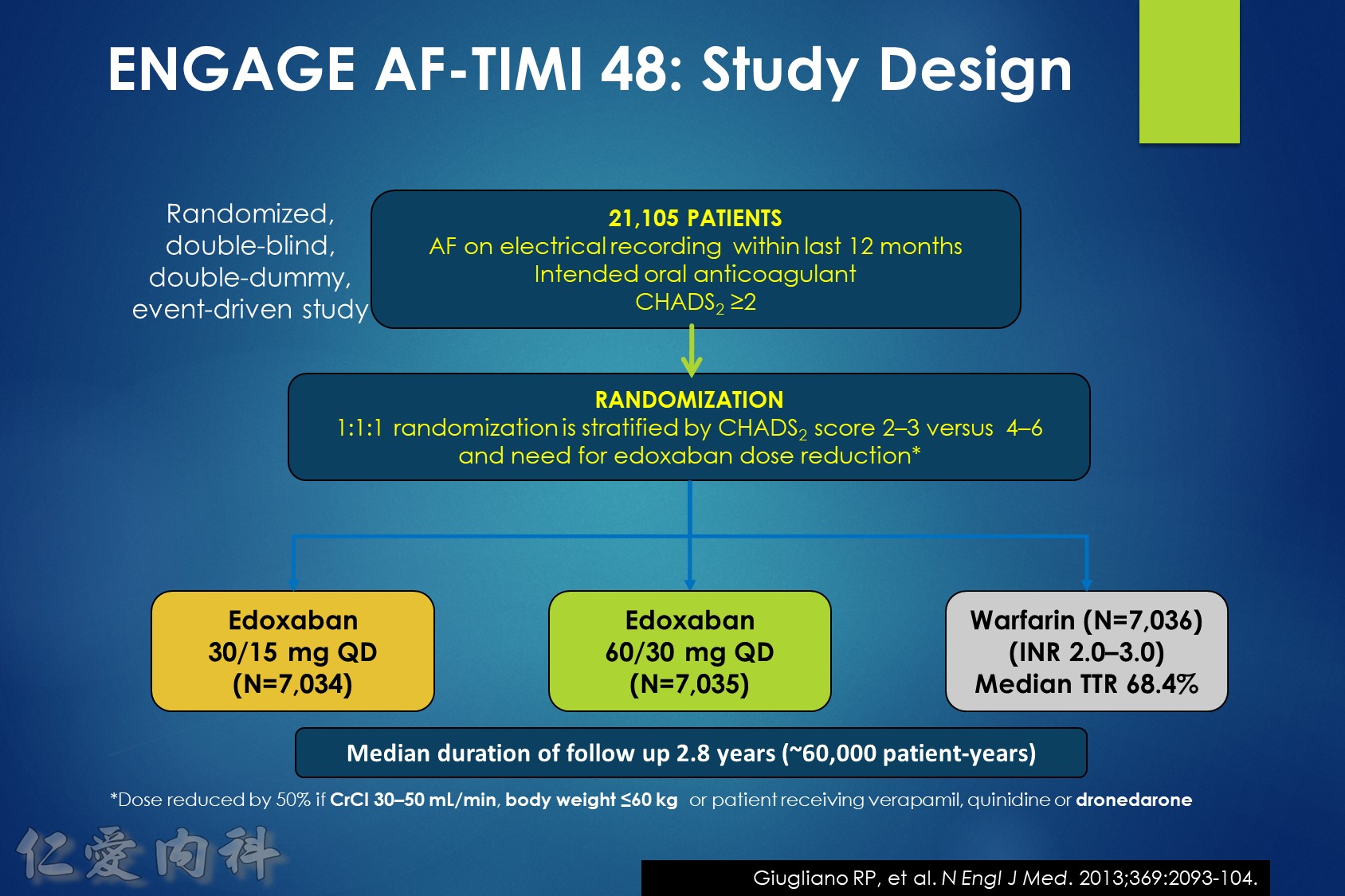
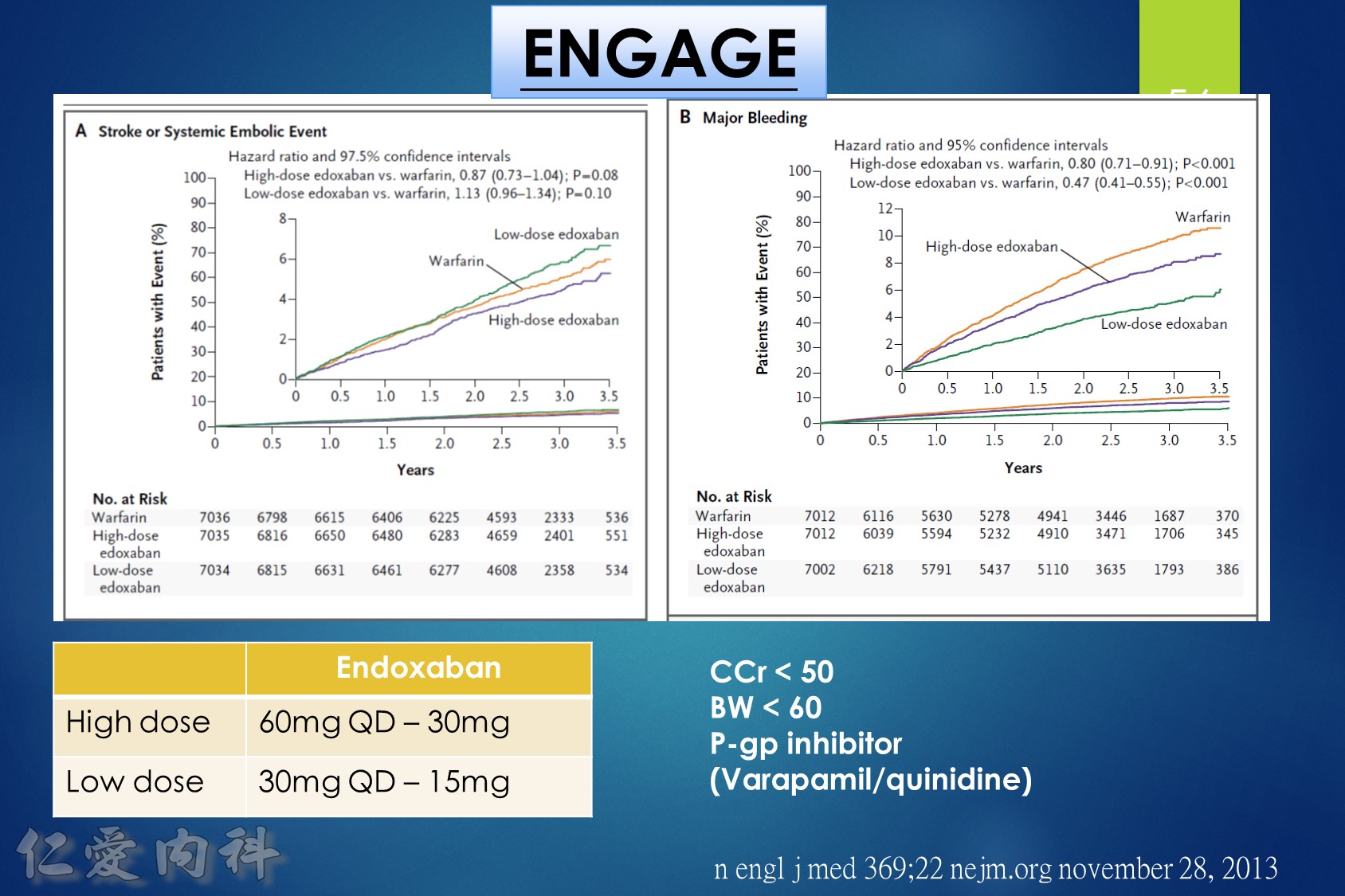
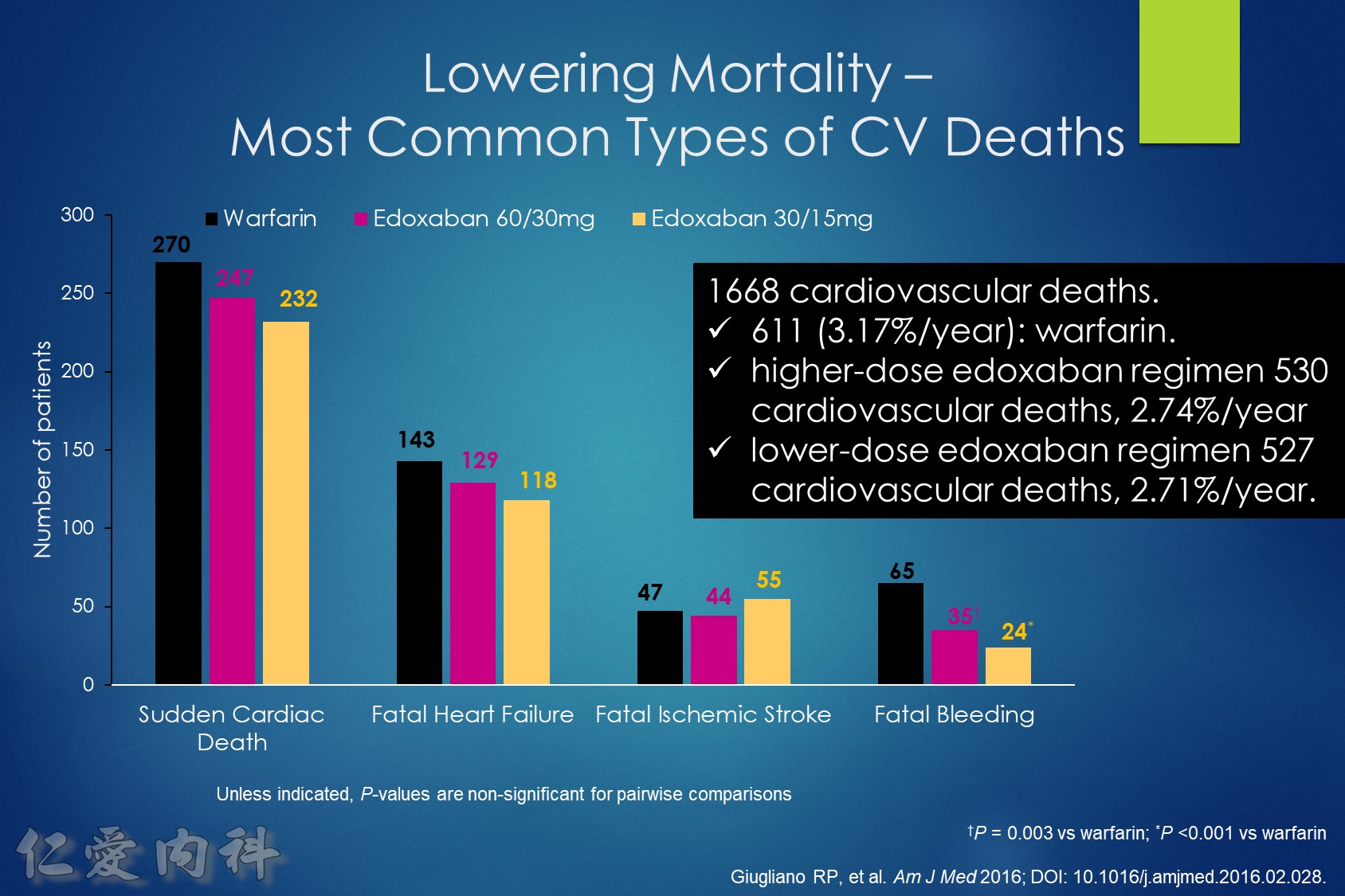
In patients with atrial fibrillation at moderate to high risk of stroke randomized to edoxaban vs. warfarin in the ENGAGE AF-TIMI 48 trial, the significant reduction in cardiovascular mortality observed with edoxaban was mediated primarily by lower rates of bleeding that were either fatal or contributed to death in the subsequent 30 days.